
Game-Changing Technology for the Vaccine
and Therapeutic Biologics Business
Noble Financial Sixth Annual Equity Conference
ONTRACK 2010

This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are not guarantees of future performance and are subject to risks and uncertainties that are difficult to predict and which may cause our actual results and performance to differ materially from those expressed or forecasted in any such forward-looking statements. These risks and uncertainties are discussed in our registration statement on file with the Securities and Exchange Commission. Unless required by law, the company undertakes no obligation to update publicly any forward-looking statements.
SAFE HARBOR STATEMENT
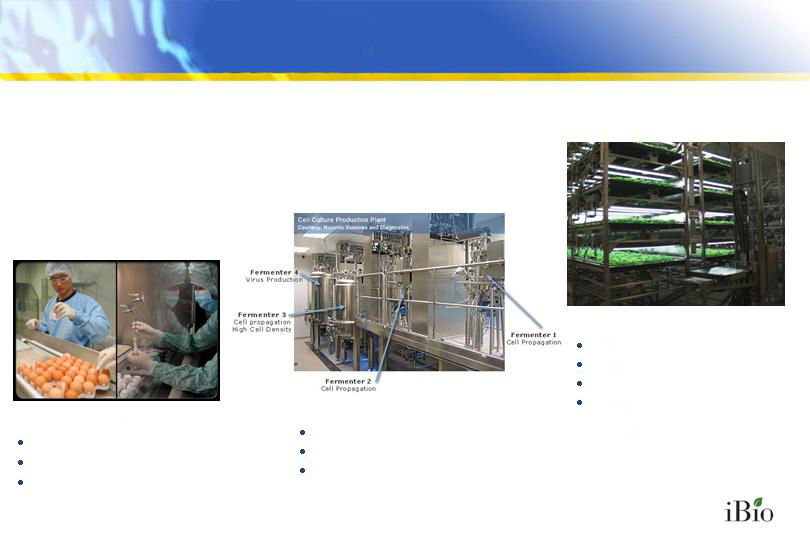
iBioLaunch Platform: Best Technology for New
Influenza Vaccines
Photo from Medicinenet.com
Traditional: Egg Technology
Slow
Expensive
Some Flu Viruses Kill Eggs
Photo from Novartis
Newer: Animal Cell Technology
Expensive Facilities
High Cost of Goods
Contamination Concerns
Best: iBioLaunch Technology
Rapid Production Cycles
Low Capital and COGs
Surge Capacity
No Animal/Human Pathogens

How Will iBio Create Shareholder Value?
High-Reward / Low-Risk Business Strategy
Broadly-sourced license revenue from defined product categories and
geographic regions – corporations & governments
Value-enhancing early-stage development and co-development of products
Based on Disruptive iBioLaunch™ Platform Technology
Significant cost, time, safety and freedom-to-operate advantages
With Substantial Non-dilutive Financing
Over $70 million invested to date by government agencies and NGOs
$15 million pilot plant funded by U.S. government
New third-party commitments of $9.8 million for malaria, $4.4 million for H1N1
flu, and $5.3 million for anthrax-plague
External funding committed for two Phase 1 clinical trials – 2010

A Substantial Business Opportunity:
Address International Needs for a Flexible and
Efficient Vaccine and Protein Manufacturing Platform
“For generations, the United States has neglected to nurture
the technologies and systems needed to respond to
emergencies related to disease. Nowhere has this been more
evident than in the response to H1N1.”
“In six to nine months last year, the United States was able
to identify this new H1N1 virus, make vaccine and begin
distributing it, though in inadequate amounts. There
is no
other disease to which our public health infrastructure could
respond anywhere near as quickly. For most new diseases,
the response time would be more like six to nine years.”
Bob Graham & Jim Talent
Washington Post, January 4, 2010
“Some analysts state that the
availability of more expensive,
state-of-the-art technological
services and
new drugs fuel health
care spending not only because the
development costs
of these products
must be recouped by industry but
also because they generate
consumer demand for more intense,
costly services even if they are not
necessarily cost-effective.”
Kaiser Family Foundation,
March 2010, citing
Congressional Budget Office

iBio’s Proprietary Technology Enables Flexible and
Efficient Bio-Product Manufacturing
iBio Opportunities: Vaccines and Bio-defense
Corporations seek entry into lucrative vaccine markets
Nations seek regional autonomy for response to outbreaks and/or
bio-defense
iBio Opportunities: Therapeutic Proteins
Corporations seek to enter orphan medical markets
Corporations seek to launch “bio-similar” or “bio-better” products

Problem: Nations Are Poorly Prepared
for Pandemics
“Pandemics are global but political calculation to confront them is decidedly
local,” Yanzhong Huang, YaleGlobal – September 2009
Federal officials predicted that 160 million doses of H1N1 vaccine would be
available by October. In reality, there were fewer
than 30 million by that
time. Former U.S. Senators Bob Graham (D-Florida) and Jim Talent (R-
Missouri) wrote a scathing January 4 editorial blaming the shortage on
U.S.
failure to “nurture the technologies and systems needed.” – CSIS, January
2010
“Recent clinical, epidemiological and laboratory evidence suggests that the
impact of a pandemic caused by the current H5N1 strain would be similar to
that of the 1918-19
pandemic,” Michael T. Osterholm, Ph.D., MPH, Director,
Center for Infectious Disease Research and Policy, 2008

Problem: Bio-Terrorist Attacks Are Inevitable
Washington Post –
December 29, 2004 – “Only a thin
wall of terrorist ignorance and
inexperience now protects us.” -- Richard Danzig,
former Navy Secretary and Pentagon bioterrorism
consultant
CNN – January 26, 2010
– “A commission set up to
assess national security measures on Tuesday
gave the U.S. government a failing grade in
improving response time to a biological attack.”

The iBio Solution
Deployment of the iBioLaunch™ Platform to Produce Vaccines
for Pandemic Outbreaks and Combat Bioterrorism
Dramatic Improvement in Speed of Response
Days and weeks versus months
Location Flexibility and Practical Surge Capacity
Modular design and ease of tech transfer enable regional autonomy or
decentralized manufacturing
Scalability not constrained by biological limitations of fermentation and
cell culture methods, nor by equipment fabrication
times
Substantial Reduction in Capital Requirements for Scale
Less than $100 million
capital investment targets capacity of 1.2 billion
doses per year (U.S. government-sponsored
facility)
Press reports state that a cell-based facility under development will
require
$1 billion for a capacity of only
150 million doses per year

Third Party Investments in iBioLaunch Platform
Vaccine Applications
The Bill & Melinda Gates Foundation and the Sabin Vaccine
Institute - $ 33.3 million for iBioLaunch platform applications
Avian Influenza Vaccine - $ 11.4 million
Malaria Vaccine - $ 13.3 million
Sleeping Sickness Vaccine - $ 8.2 million
Hookworm Vaccine - $ 0.4 million
U.S. Government - $ 37.4 million for iBioLaunch platform
applications
Accelerated Manufacturing Program - $ 14.8 million
H1N1 Influenza Vaccine - $ 4.4 million
Plague-Anthrax Combination Vaccine - $ 18.2 million

iBio Opportunities:
Corporations Entering Vaccine Markets &
Nations Recognizing
Pandemic Disease and Bioterrorism as
National Security Threats
1. iBioLaunch technology superior for recombinant vaccines in comparison to
bioreactor based systems
No animal components or human pathogens
Lower cost of goods
Rapid batch production cycles
Excellent surge capacity
Not dependent on access to culturable pathogens
2. iBioLaunch technology can be rapidly implemented at substantially lower capital
costs than conventional methods for vaccine active ingredient manufacturing
10 to 15 percent in comparison to animal cell / virus culture methods

The Market for New and Expanded Vaccine
and Bio-defense Applications
Global vaccine market projected at $32 billion in 2012
Influenza vaccine market projected > $4 billion in 2010
Bio-defense Vaccines
U.S. spending for 2010 approximately $5 billion
Tropical Diseases
Gates Global Access Agreement
Malaria is endemic in 117 countries (2.5 billion people)
Hookworm infects approximately 700 million people worldwide
iBio is the preferred tech transfer supplier and has right of first refusal to
manufacture

iBio Opportunities:
Corporations Developing Protein Therapeutics
Orphan Medical Products
iBioLaunch technology flexibility makes feasible addressing small population,
high-value proteins
Bio-similar and Bio-better Products
iBioLaunch technology is applicable to all currently marketed biotech
therapeutic proteins
iBioLaunch technology can be used to rapidly evaluate and develop
proprietary and improved derivatives of existing products
Capital and cost of goods advantage over standard methods, making iBio a
partner of choice for new biologics being developed by others

The iBio Solution
Advantages of iBioLaunch™ Technology For Therapeutic Protein
Manufacturing
Best Economy of Production
Reduced cost of materials, More efficient space utilization, Lower process
costs, and Reduced amortization and depreciation expenses
Facility Expansion Efficiency
Time frame to bring new facilities online dramatically shorter than
conventional methods –
12 to 18 months versus 4
to 7 years
Broad Product Applicability
Proven effective for production of monoclonal antibodies
and other classes of therapeutic proteins, both proprietary and generic
Freedom to Operate
Bioreactor & cell line patents do not apply
Safety
Eliminates risk of live virus culture and animal-based components

Achievements of iBioLaunch Technology in
Therapeutic Protein Applications:
Efficient production of monoclonal antibodies, including
proprietary product candidates for influenza and anthrax
Proven applicable to interferons, growth factors, therapeutic
enzymes, insulin, lectins, protease inhibitors, nucleases,
etc.
Functional and structural properties of iBioLaunch-produced
proteins are the same as the native forms
Disulfide linkages
Glycan occupancy
Tissue distribution in animal studies
In vivo activity in animal studies
Immunogenicity (and absence thereof)

Market(s) for iBio Relevant Protein Therapeutics
Orphan Drug Opportunities
Entry enhanced by 50% orphan drug development cost U.S. tax credit
Novartis alone sells over $8 billion in orphan drugs
2,116 orphan drug designations approved by the FDA since 1983
Pfizer – Protalix deal validates big pharma interest
Global market projected at $81 billion next year
Bio-similars and Bio-betters
Biologics market is growing at double the pace of the pharmaceutical industry
This rapid growth is fueling fears regarding affordability
A number of biological drugs are losing patent protection
Companies developing bio-generics are in two main groups
Divisions of established generics manufacturers
Research-based biotech companies
Current market estimated by Deutsche Bank at $26 billion

Intellectual Property
Product Filings (U.S. and International)
Influenza vaccines
HPV vaccines
Influenza monoclonal antibodies
Anthrax vaccines
Plague vaccine
Trypanosomiasis and Malaria vaccines
Technology Filings (U.S. and International)
Virus-induced gene silencing in plants
Activation of transgenes in plants by viral vectors
Production of proteins in plants with launch vector
Transient expression of proteins in plants
Protein expression in seedlings and clonal root cultures
Thermostable carrier molecule

Fraunhofer USA CMB – iBio Relationship
Fraunhofer USA CMB is a not-for-profit R&D organization
Develops plant-based technologies to develop and manufacture vaccines
and therapeutic biologics
Specialists in vaccine development, plant molecular biology, immunology,
and protein engineering
Fraunhofer USA CMB receives grants and contracts from U.S.
DoD, Bill & Melinda Gates Foundation and iBio, Inc.
iBio owns the technology developed with all sources of funding
Worldwide exclusive rights in human healthcare
Cooperation with Gates on “Global Access” in exchange for rights of first
refusal on tech transfer contracts and contract manufacturing

Synergy of iBio-CMB Collaboration
Enhances Rate of Technical Innovation
Non-dilutive and highly accretive
collaboration with Fraunhofer USA CMB
Leveraged funding for technology / product
development >$70 million through 2010
Dec. 2009 - $9.85 million from Bill & Melinda
Gates Foundation for malaria; Ongoing
funding for avian influenza vaccine clinicals
Mar. 2010 - $4.4 million from DARPA for
H1N1 influenza vaccine
Apr. 2010 - $5.3 million from DTRA for
combination anthrax-plague vaccine
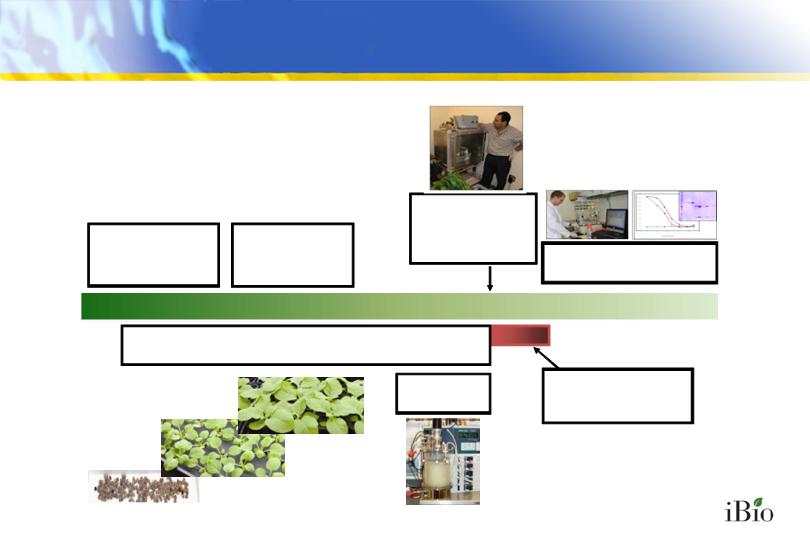
Design &
synthesize
target gene
Clone target gene
into “launch
vector”
Harvest biomass &
recover target protein
Infiltrate with
agrobacteria
containing launch
vectors
Grow plants hydroponically
iBioLaunch™
Accelerated High Capacity
Production of
Pharmaceutical Proteins
Grow
agrobacteria
Target protein
accumulates
in infiltrated plants

Management Team and Directors
Management
Robert B. Kay - CEO & Executive Chairman
Robert Erwin - President
Frederick Larcombe, CPA – Interim CFO
Vidadi Yusibov, Ph.D. - Chief Scientific Officer
Board of Directors
Robert B. Kay – Executive Chairman
Dr. Pamela Bassett – Cantor Fitzgerald
Glenn Chang – First American International Bank
General James T. Hill (ret.) – The J.T. Hill Group
John D. McKey Jr. – McCarthy, Summers, Bobko, Wood, Sawyer & Perry
General (ret.) Philip K. Russell, M.D. – Sabin Institute

iBio Valuation Metrics
Industry Data
Median Upfront Payments in Post-Phase 1 Clinical Development: $20 Million (27 deals -
ReCap)
Product Royalties: Preclinical 0 to 5%; Clinical 5 to 10%, Launched Products 8 to 20%
Platform Royalties: Up to 11.7%
Addressable Markets: Total Approximately $100 Billion
Influenza: $4 billion
Other vaccines: $30 billion
Biogenerics: Top 8 biologics worldwide sales of $26 billion
Orphan Biologics: 50% of orphan drug total of $81 billion: $40 billion
Imputed iBio Value
For each 1% Market Penetration, with just a 5% Average Royalty, iBio Annual Revenues
Would Equal $50 million
(Almost All of Which Would Fall to Bottom Line)
Current PE ratio of exchange-traded biotech basket fund, BBH, is 14X
At PE of 14X, market cap would equal $700 Million per 1% Market Penetration (without
including possible upfront
fees)

Recap: iBio’s Value Proposition
Diversified licensing approach to commercialize proprietary
platform
Less capital required vs. single-product biotech
Non-dilutive financing enhances value
Superior technology with third party validation in hand
Large and growing markets
Early and low-risk revenue
Stock is currently undervalued in comparison to peers

Financial Metrics
Cash and Equivalents: $1.5 million (as of 3/31/10)
No Debt
Stock Price: (OTCBB: IBPM) $1.02 on 5/28/10
Market-Cap: $28.8 Million
Shares Outstanding: 28.3 million
Float: 14.2 million

9 Innovation Way, Suite 100
Newark, DE 19711
www.ibioinc.com
Contact: Investor Contact: Media Contact:
Robert Erwin Jeffrey Benison Melody A. Carey
President Managing Director Co-President
iBio, Inc. Little Gem Life Science Partners Rx Communications Group
302-355-2335 212-334-8709 917-322-2571
rerwin@ibioinc.com jeffrey@littlegem.us mcarey@rxir.com