Exhibit 99.1

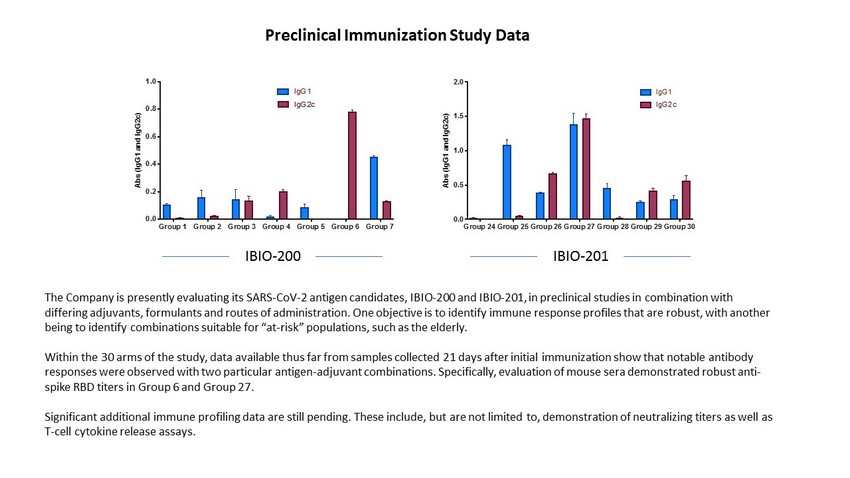
Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 0.0 0.2 0.4 0.6 0.8 1.0 A b s ( I g G 1 a n d I g G 2 c ) IgG1 IgG2c Group 24Group 25Group 26Group 27Group 28Group 29Group 30 0.0 0.5 1.0 1.5 2.0 A b s ( I g G 1 a n d I g G 2 c ) IgG1 IgG2c IBIO - 200 IBIO - 201 The Company is presently evaluating its SARS - CoV - 2 antigen candidates, IBIO - 200 and IBIO - 201, in preclinical studies in combinat ion with differing adjuvants, formulants and routes of administration. One objective is to identify immune response profiles that are rob ust, with another being to identify combinations suitable for “at - risk” populations, such as the elderly. Within the 30 arms of the study, data available thus far from samples collected 21 days after initial immunization show that not able antibody responses were observed with two particular antigen - adjuvant combinations. Specifically, evaluation of mouse sera demonstrated robust anti - spike RBD titers in Group 6 and Group 27. Significant additional immune profiling data are still pending. These include, but are not limited to, demonstration of neutr ali zing titers as well as T - cell cytokine release assays. Preclinical Immunization Study Data