
Exhibit 99.1

Growing A Next - Gen Biologics Pipeline CORPORATE PRESENTATION: August 2021 Tom Isett, Chairman & CEO

Certain statements in this presentation constitute "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended . Words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," "predict," "forecast," "project," "plan," "intend" or similar expressions, or statements regarding intent, belief, or current expectations, are forward - looking statements . These forward - looking statements are based upon current estimates . While the Company believes these forward - looking statements are reasonable, undue reliance should not be placed on any such forward - looking statements, which are based on information available to us on the date of this presentation . These forward - looking statements are subject to various risks and uncertainties, many of which are difficult to predict that could cause actual results to differ materially from current expectations and assumptions from those set forth or implied by any forward - looking statements . Important factors that could cause actual results to differ materially from current expectations include, among others, the Company’s ability to obtain regulatory approvals for commercialization of its product candidates, including its COVID - 19 vaccines and RTX - 003 , or to comply with ongoing regulatory requirements, regulatory limitations relating to its ability to promote or commercialize its product candidates for specific indications, acceptance of its product candidates in the marketplace and the successful development, marketing or sale of products, its ability to maintain its license agreements, the continued maintenance and growth of its patent estate, its ability to establish and maintain collaborations, its ability to obtain or maintain the capital or grants necessary to fund its research and development activities, competition, its ability to retain its key employees or maintain its NYSE American listing, and the other factors discussed in the Company’s most recent Annual Report on Form 10 - K and the Company’s subsequent filings with the SEC, including subsequent periodic reports on Forms 10 - Q and 8 - K . The information in this presentation is provided only as of today, and we undertake no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law . This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, nor will there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such state or jurisdiction . 2 Forward - Looking Statements
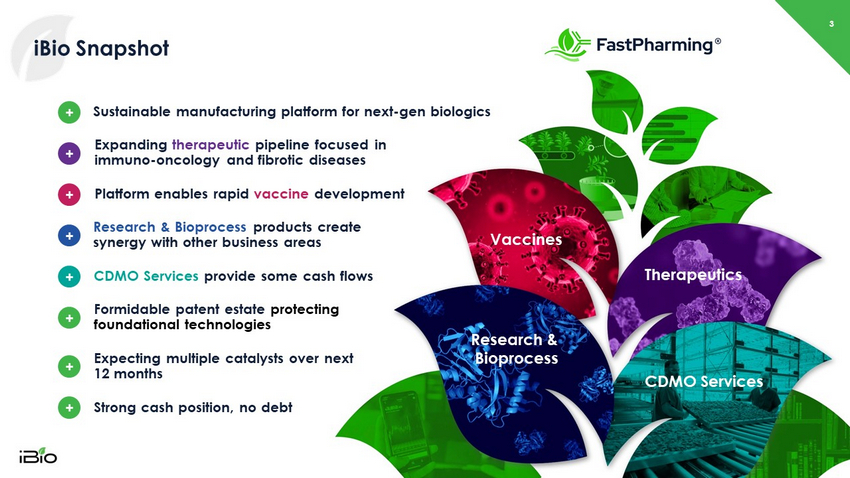
iBio Snapshot Vaccines Therapeutics CDMO Services Research & Bioprocess 3 CDMO Services provide some cash flows + Expanding therapeutic pipeline focused in immuno - oncology and fibrotic diseases + Platform enables rapid vaccine development + Research & Bioprocess products create synergy with other business areas + + Sustainable manufacturing platform for next - gen biologics + Strong cash position, no debt + Formidable patent estate protecting foundational technologies + Expecting multiple catalysts over next 12 months
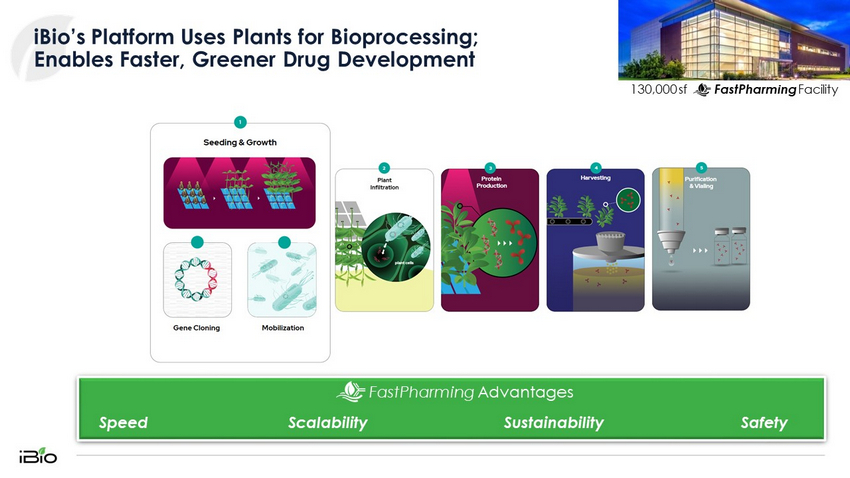
4 iBio’s Platform Uses Plants for Bioprocessing; Enables Faster, Greener Drug Development 130,000 sf FastPharming Facility Sustainability FastPharming Advantages Scalability Speed Safety
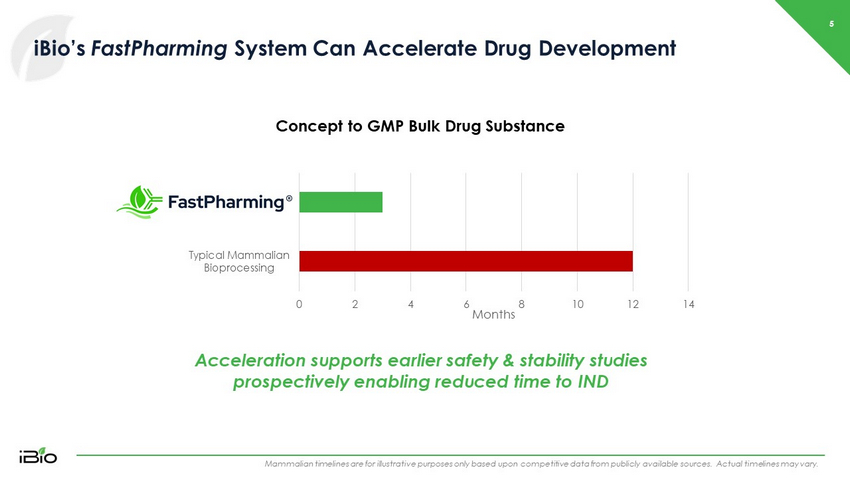
5 iBio’s FastPharming System Can Accelerate Drug Development Mammalian timelines are for illustrative purposes only based upon competitive data from publicly available sources. Actual t ime lines may vary. Concept to GMP Bulk Drug Substance Acceleration supports earlier safety & stability studies prospectively enabling reduced time to IND 0 2 4 6 8 10 12 14 Typical Mammalian Bioprocessing Months
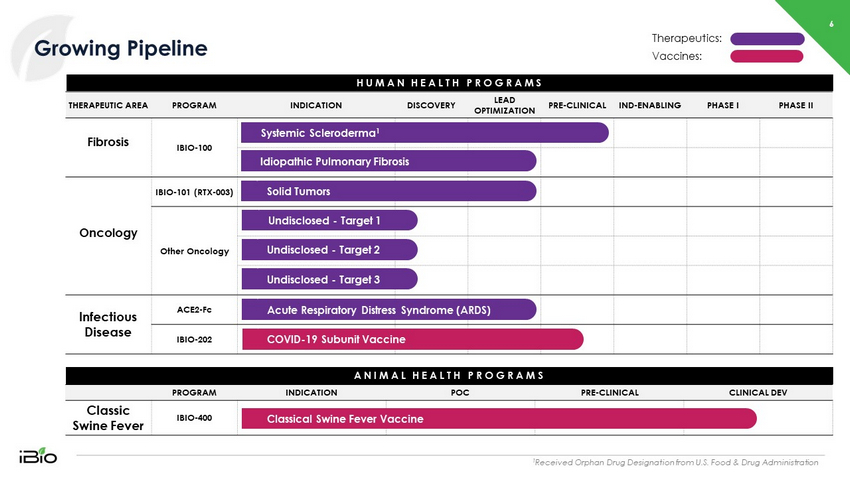
6 Growing Pipeline 1 Received Orphan Drug Designation from U.S. Food & Drug Administration HUMAN HEALTH PROGRAMS PROGRAM INDICATION POC PRE - CLINICAL CLINICAL DEV Classic Swine Fever IBIO - 400 THERAPEUTIC AREA PROGRAM INDICATION DISCOVERY LEAD OPTIMIZATION PRE - CLINICAL IND - ENABLING PHASE I PHASE II Fibrosis IBIO - 100 Oncology IBIO - 101 (RTX - 003) Other Oncology Infectious Disease ACE2 - Fc IBIO - 202 ANIMAL HEALTH PROGRAMS Classical Swine Fever Vaccine Acute Respiratory Distress Syndrome (ARDS) COVID - 19 Subunit Vaccine Idiopathic Pulmonary Fibrosis Systemic Scleroderma 1 Undisclosed - Target 2 Undisclosed - Target 1 Undisclosed - Target 3 Solid Tumors Therapeutics: Vaccines:
Therapeutics Oncology Fibrosis Infectious Diseases
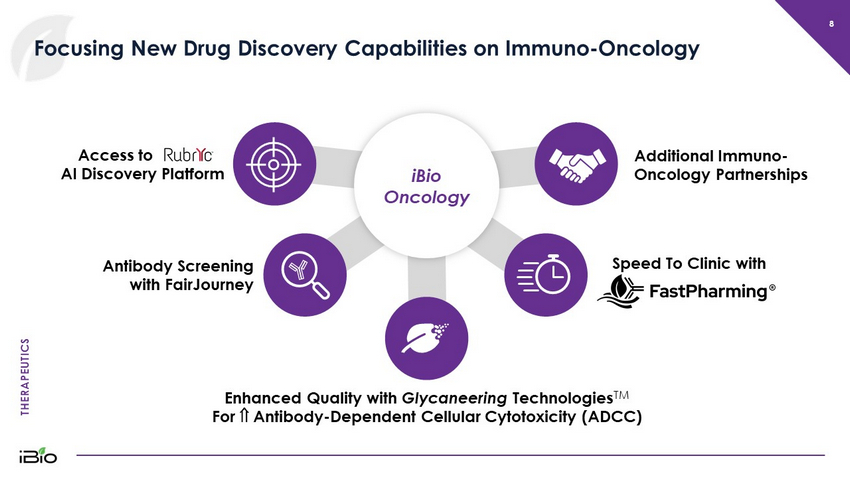
THERAPEUTICS 8 Focusing New Drug Discovery Capabilities on Immuno - Oncology Speed To Clinic with Enhanced Quality with Glycaneering Technologies TM For Antibody - Dependent Cellular Cytotoxicity (ADCC) Antibody Screening with FairJourney Access to RubrYc’s AI Discovery Platform iBio Oncology Additional Immuno - Oncology Partnerships
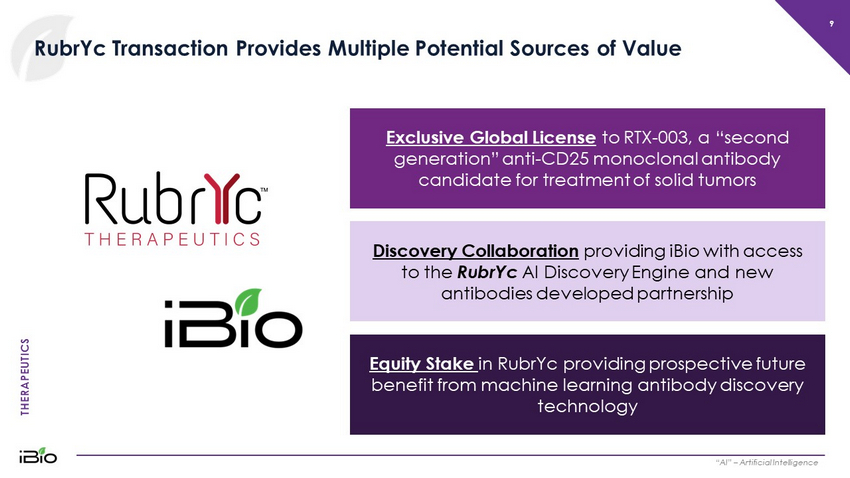
THERAPEUTICS 9 RubrYc Transaction Provides Multiple Potential Sources of Value “AI” – Artificial Intelligence Exclusive Global License to RTX - 003, a “second generation” anti - CD25 monoclonal antibody candidate for treatment of solid tumors Equity Stake in RubrYc providing prospective future benefit from machine learning antibody discovery technology Discovery Collaboration providing iBio with access to the RubrYc AI Discovery Engine and new antibodies developed partnership
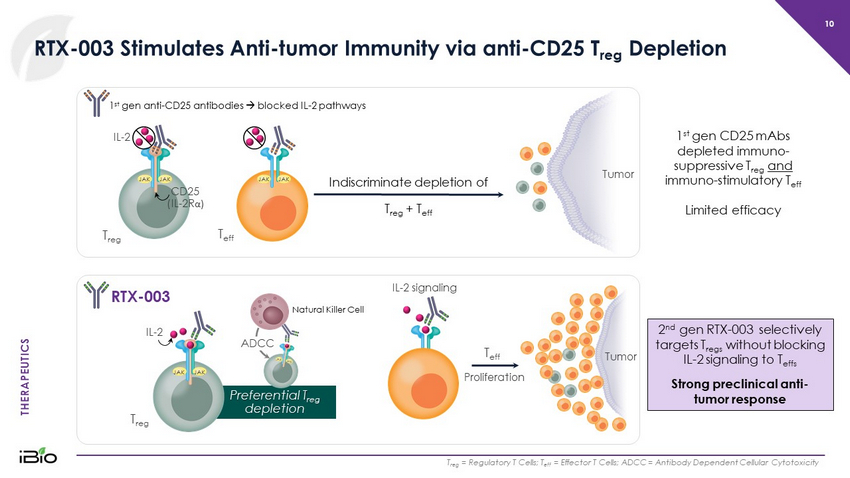
THERAPEUTICS Natural Killer Cell Indiscriminate depletion of T reg + T eff RTX - 003 Stimulates Anti - tumor Immunity via anti - CD25 T reg Depletion 10 T reg = Regulatory T Cells; T eff = Effector T Cells; ADCC = Antibody Dependent Cellular Cytotoxicity T eff JAK JAK 1 st gen anti - CD25 antibodies blocked IL - 2 pathways RTX - 003 JAK JAK Tumor Tumor T eff T reg JAK JAK Preferential T reg depletion 1 st gen CD25 mAbs depleted immuno - suppressive T reg and immuno - stimulatory T eff Limited efficacy 2 nd gen RTX - 003 selectively targets T regs without blocking IL - 2 signaling to T effs Strong preclinical anti - tumor response IL - 2 CD25 (IL - 2R α ) JAK JAK IL - 2 T reg IL - 2 signaling Proliferation ADCC
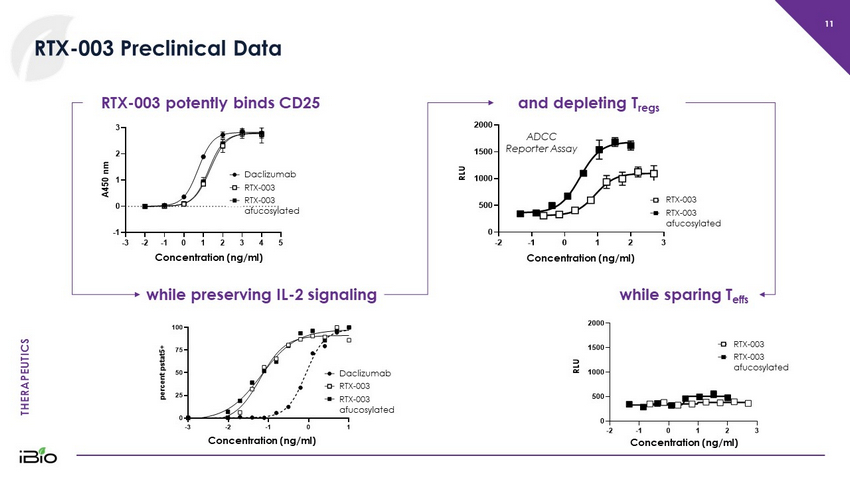
THERAPEUTICS -2 -1 0 1 2 3 0 500 1000 1500 2000 Tregs Log [Ab], (ng/mL) R L U D11 D11 Afucosylated 11 RTX - 003 Preclinical Data while preserving IL - 2 signaling -3 -2 -1 0 1 0 25 50 75 100 Log [IL-2] (ng/mL) p e r c e n t p s t a t 5 + Dac hD11 wt hD11 Afucosylated Daclizumab RTX - 003 RTX - 003 afucosylated -3 -2 -1 0 1 2 3 4 5 -1 0 1 2 3 Log [Ab] (ng/mL) A 4 5 0 n m Daclizumab hD11 WT hD11 Afucosylated RTX - 003 potently binds CD25 Daclizumab RTX - 003 RTX - 003 afucosylated Concentration (ng/ml) Concentration (ng/ml) and depleting T regs ADCC Reporter Assay while sparing T effs RTX - 003 RTX - 003 afucosylated Concentration (ng/ml) RTX - 003 RTX - 003 afucosylated Concentration (ng/ml) RLU RLU
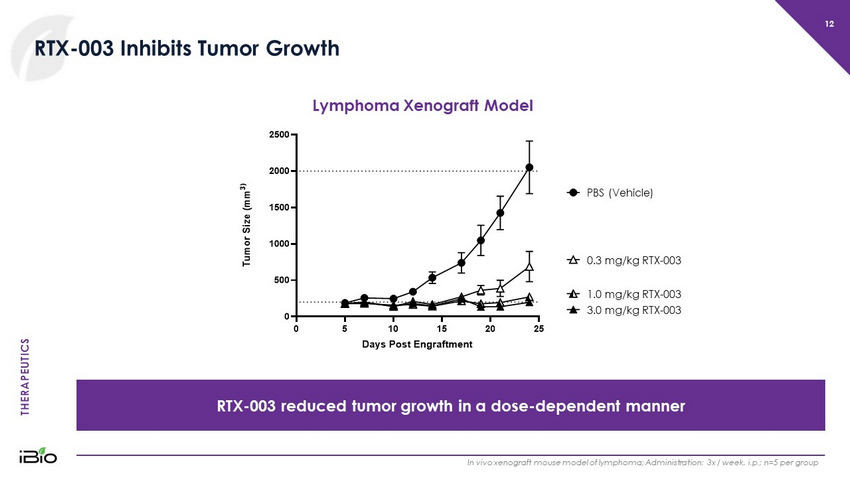
THERAPEUTICS 12 RTX - 003 Inhibits Tumor Growth RTX - 003 reduced tumor growth in a dose - dependent manner In vivo xenograft mouse model of lymphoma; Administration: 3x / week, i.p.; n=5 per group PBS (Vehicle) 0.3 mg/kg RTX - 003 1.0 mg/kg RTX - 003 3.0 mg/kg RTX - 003 Lymphoma Xenograft Model
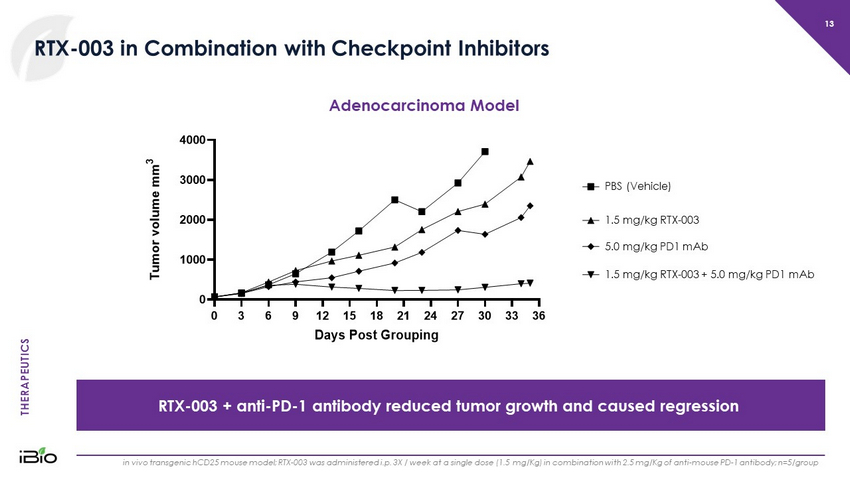
THERAPEUTICS 13 RTX - 003 in Combination with Checkpoint Inhibitors RTX - 003 + anti - PD - 1 antibody reduced tumor growth and caused regression in vivo transgenic hCD25 mouse model; RTX - 003 was administered i.p. 3X / week at a single dose (1.5 mg/Kg) in combination with 2 .5 mg/Kg of anti - mouse PD - 1 antibody; n=5/group Adenocarcinoma Model 0 3 6 9 12 15 18 21 24 27 30 33 36 0 1000 2000 3000 4000 Days Post Grouping T u m o r v o l u m e m m 3 PD1 5 mg/kg D11 1.5 + PD1 5 mg/kg PBS D11 1.5 mg/kg PBS (Vehicle) 1.5 mg/kg RTX - 003 5.0 mg/kg PD1 mAb 1.5 mg/kg RTX - 003 + 5.0 mg/kg PD1 mAb
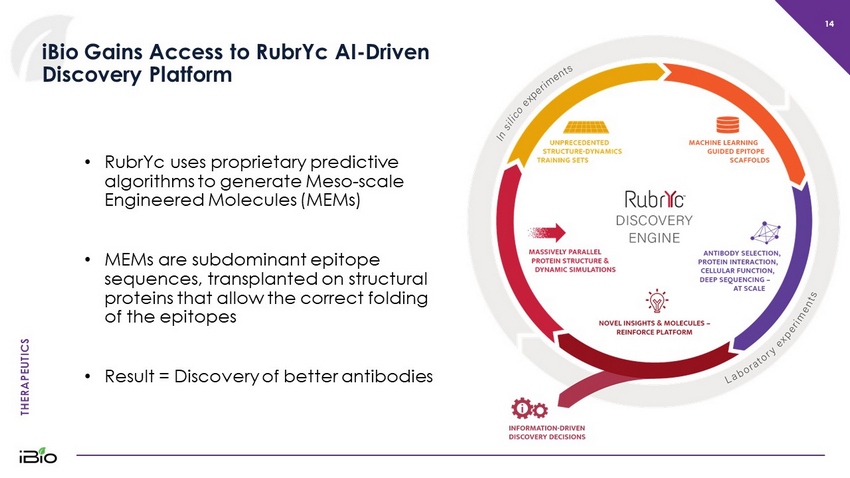
THERAPEUTICS 14 iBio Gains Access to RubrYc AI - Driven Discovery Platform • RubrYc uses proprietary predictive algorithms to generate Meso - scale Engineered Molecules (MEMs ) • MEMs are subdominant epitope sequences, transplanted on structural proteins that allow the correct folding of the epitopes • Result = Discovery of better antibodies
THERAPEUTICS 15 Fibrotic & Infectious Diseases IBIO - 100 for the treatment of fibrotic disorders ACE2 - Fc as a prospective COVID - 19 program
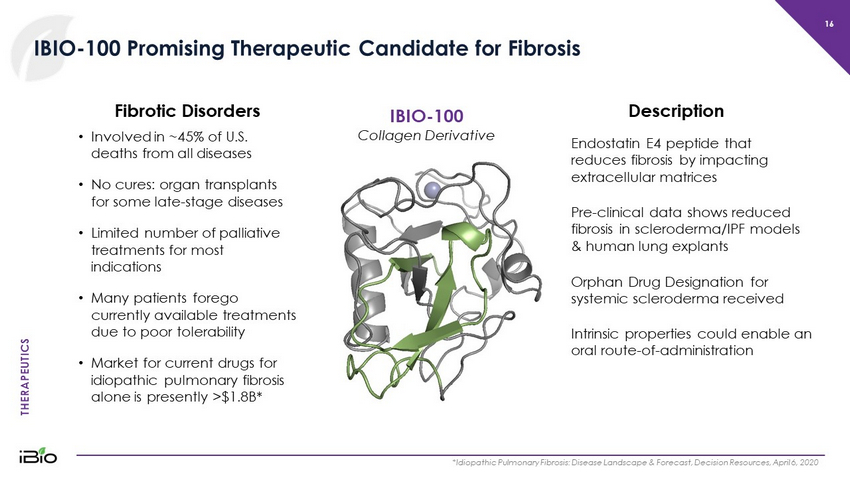
THERAPEUTICS 16 IBIO - 100 Promising Therapeutic Candidate for Fibrosis *Idiopathic Pulmonary Fibrosis: Disease Landscape & Forecast, Decision Resources, April 6, 2020 IBIO - 100 Collagen Derivative • Involved in ~45% of U.S. deaths from all diseases • No cures: organ transplants for some late - stage diseases • Limited number of palliative treatments for most indications • Many patients forego currently available treatments due to poor tolerability • Market for current drugs for idiopathic pulmonary fibrosis alone is presently >$1.8B* Fibrotic Disorders Endostatin E4 peptide that reduces fibrosis by impacting extracellular matrices Pre - clinical data shows reduced fibrosis in scleroderma/IPF models & human lung explants Description Orphan Drug Designation for systemic scleroderma received Intrinsic properties could enable an oral route - of - administration
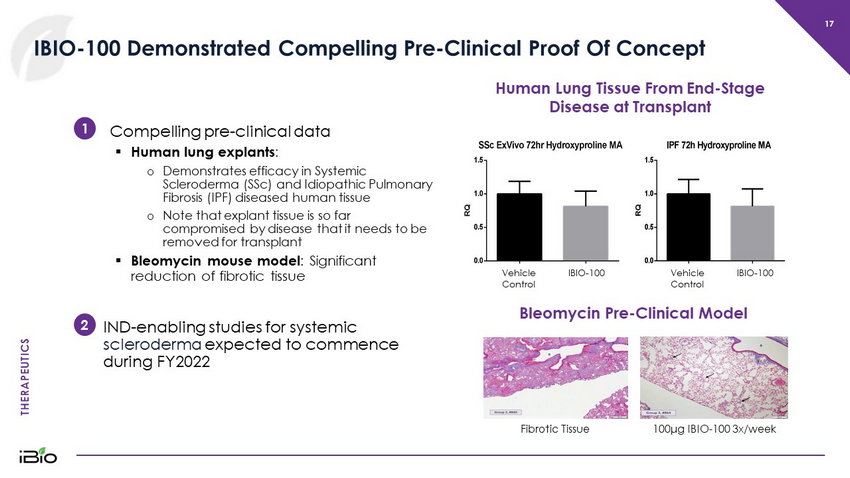
THERAPEUTICS 17 IBIO - 100 Demonstrated Compelling Pre - Clinical Proof Of Concept Compelling pre - clinical data ▪ Human lung explants : o D emonstrates efficacy in Systemic Scleroderma (SSc ) and Idiopathic Pulmonary Fibrosis (IPF ) diseased human tissue o Note that explant tissue is so far compromised by disease that it needs to be removed for transplant ▪ Bleomycin mouse model : Significant reduction of fibrotic tissue IND - enabling studies for systemic scleroderma expected to commence during FY2022 Human Lung Tissue From End - Stage Disease at Transplant Vehicle Control IBIO - 100 Vehicle Control IBIO - 100 100 μ g I BIO - 100 3x/week Fibrotic Tissue Bleomycin Pre - Clinical Model 1 2
THERAPEUTICS 18 ACE - F2C: Potential COVID - 19 Therapeutic I B IO I N - L ICENSED W ORLDWIDE R IGHTS FOR C ORONAVIRIDAE • ACE2 - Fc is a recombinant protein comprised of human angiotensin converting enzyme 2 (ACE2) fused to a human immunoglobulin G Fc fragment (Fc) • Molecule targets the coronavirus virions by using the ACE2 extracellular domain to bind the spike protein and block infection of healthy cells, while the fused Fc domain prolongs the life of the protein in circulation • Benefits of a traditional neutralizing antibody, while prospectively limiting the potential for “viral escape” • Planet’s in vitro studies demonstrated that ACE2 - Fc blocks SARS - CoV - 2 virus from infecting Vero E6 cells • Continuing to assess the regulatory and competitive landscape Source: NEXU Science Communication | Reuters
Vaccines COVID - 19 Classic Swine Fever
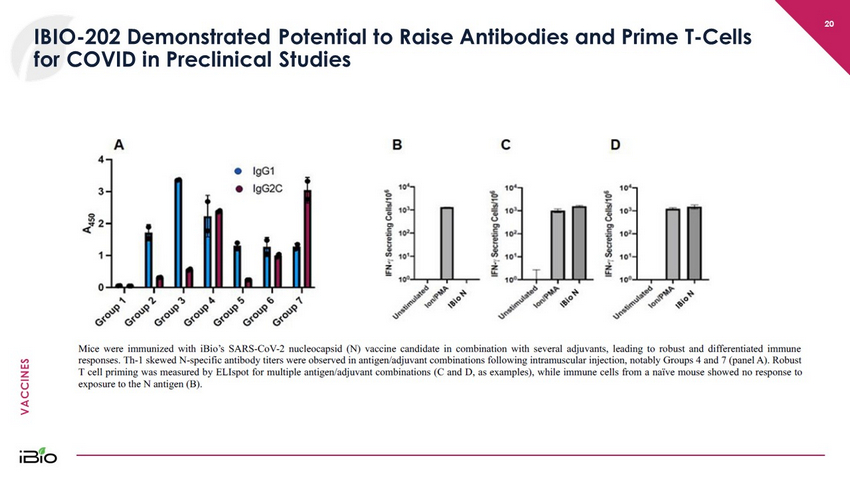
VACCINES IBIO - 202 Demonstrated Potential to Raise Antibodies and Prime T - Cells for COVID in Preclinical Studies 20
VACCINES 21 IBIO - 202 Could Be Complementary To Current and Future S - Protein Vaccines Adjuvanted to potentially allow for greater immunogenicity and/or dose sparing An “N - only” vaccine may be highly complementary to existing first - generation, S protein – directed vaccines Prospectively suitable for delivery via routes other than intramuscular injection The antigen is produced in our rapidly scalable FastPharming System The N - protein: • is abundantly expressed during infection • contains immunogenic epitopes • is more highly conserved than the S protein among the viral variants – new viral variants may be less likely to escape vaccine protection if vaccines include conserved sequences. 1,2,3,4 • is significantly more effective than S protein in stimulating antibody - dependent natural killer cell activation, a critical element of the adaptive immune response that the SARS - CoV - 2 virus attempts to evade 5 1. Zhao, P. et al. Immune responses against SARS - coronavirus nucleocapsid protein induced by DNA vaccine. Virology 331, 128 – 135 (20 05) 2. Oliveira, S. C., de Magalhães, M. T. Q. & Homan, E. J. Immunoinformatic Analysis of SARS - CoV - 2 Nucleocapsid Protein and Identifi cation of COVID - 19 Vaccine Targets. Front. Immunol. 11, (2020) 3. Dutta, N. K., Mazumdar, K. & Gordy, J. T. The Nucleocapsid Protein of SARS – CoV - 2: A Target for Vaccine Development. Journal of V irology 94, (2020) 4. Dai, L. & Gao, G. F. Viral targets for vaccines against COVID - 19. Nature Reviews Immunology 21, 73 – 82 (2021) 5. Fielding CA, Sabberwal P, Williamson JC, Greenwood EJD, Crozier TWM, Zelek W, Seow J, Graham C, Huettner I, Edgeworth JD, Mor gan BP, Ladell K, Eberl M, Humphreys IR, Merrick B, Doores K, Wilson SJ Lehner PJ, Wang ECY, Stanton RJ. ADNKA overcomes SARS - CoV2 - mediated NK cell inhibition through non - spike antibodies. bioRxiv, (April 2021)
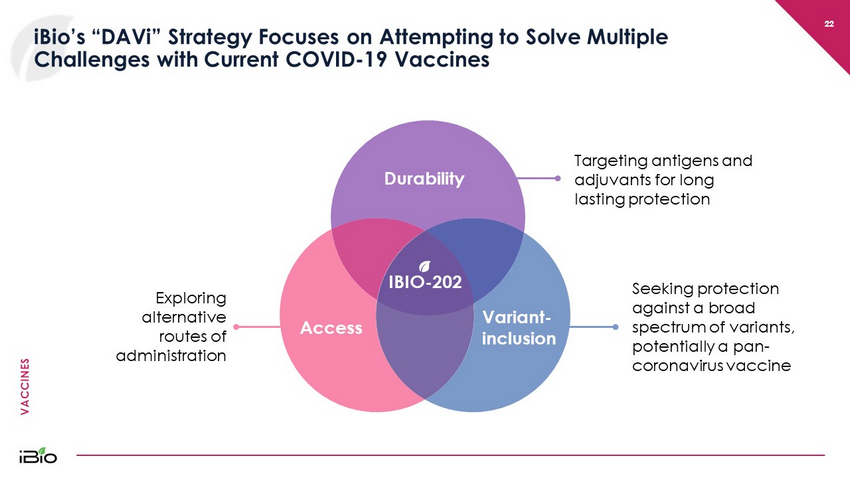
VACCINES 22 iBio’s “DAVi” Strategy Focuses on Attempting to Solve Multiple Challenges with Current COVID - 19 Vaccines 22 IBIO - 202 Targeting antigens and adjuvants for long lasting protection Exploring alternative routes of administration Seeking protection against a broad spectrum of variants, potentially a pan - coronavirus vaccine Durability Variant - inclusion Access
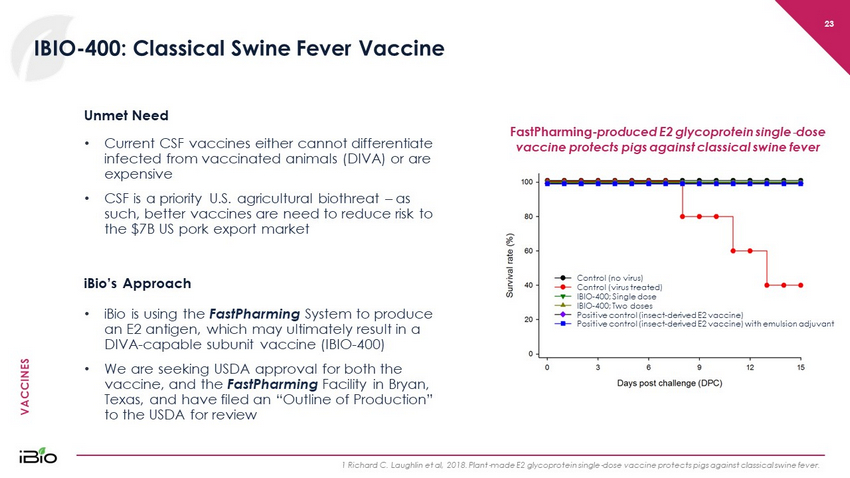
VACCINES 23 IBIO - 400: Classical Swine Fever Vaccine 1 Richard C. Laughlin et al, 2018. Plant - made E2 glycoprotein single - dose vaccine protects pigs against classical swine fever. Unmet Need iBio’s Approach • iBio is using the FastPharming System to produce an E2 antigen, which may ultimately result in a DIVA - capable subunit vaccine (IBIO - 400) • We are seeking USDA approval for both the vaccine, and the FastPharming Facility in Bryan, Texas, and have filed an “Outline of Production” to the USDA for review FastPharming - produced E2 glycoprotein single - dose vaccine protects pigs against classical swine fever • Current CSF vaccines either cannot differentiate infected from vaccinated animals (DIVA) or are expensive • CSF is a priority U.S. agricultural biothreat – as such, better vaccines are need to reduce risk to the $7B US pork export market Control (no virus) Control (virus treated) IBIO - 400; Single dose IBIO - 400; Two doses Positive control (insect - derived E2 vaccine) Positive control (insect - derived E2 vaccine) with emulsion adjuvant

Research & Bioprocess Products CDMO
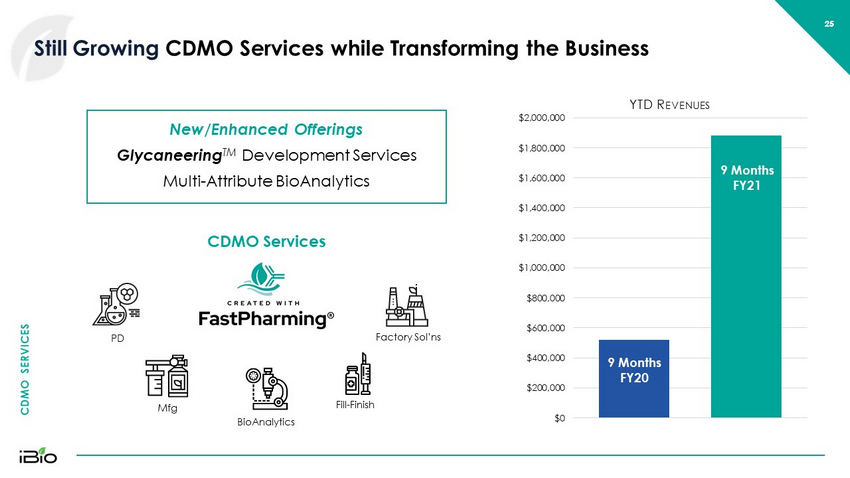
CDMO SERVICES 25 Still Growing CDMO Services while Transforming the Business 25 H1 FY21 H1 FY21 $0 $200,000 $400,000 $600,000 $800,000 $1,000,000 $1,200,000 $1,400,000 $1,600,000 $1,800,000 $2,000,000 YTD R EVENUES [= New/Enhanced Offerings Glycaneering TM Development Services Multi - Attribute BioAnalytics 9 Months FY20 9 Months FY21 CDMO Services PD Mfg BioAnalytics Fill - Finish Factory Sol’ns
26 New Research and Bioproducts Offerings Launched
Summary

iBio, By the Numbers 28 Summary Transformation of plants into high - productivity bioreactors via infiltration complimentary segments with growth opportunities Biopharm pipeline with programs + discovery capability employees (2x v. PY) revenues from relaunched CDMO services Cash reserves of fuels business into 2023

Experience Tom Isett CEO & Chairman Martin Brenner , DVM, Ph.D. CSO Robert Lutz, MBA CFBO Randy Maddux , MBA COO Lisa Middlebrook CHRO Management Team with Diverse Experience 29
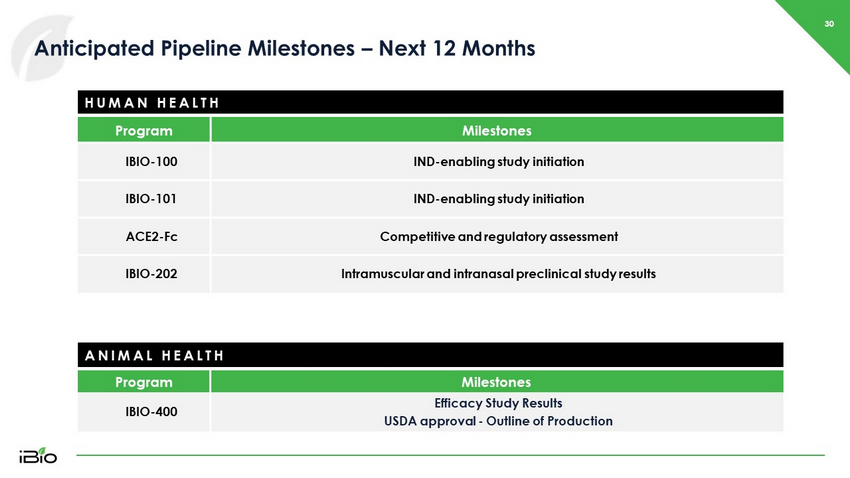
30 Anticipated Pipeline Milestones – Next 12 Months HUMAN HEALTH Program Milestones IBIO - 400 Efficacy Study Results USDA approval - Outline of Production Program Milestones IBIO - 100 IND - enabling study initiation IBIO - 101 IND - enabling study initiation ACE2 - Fc Competitive and regulatory assessment IBIO - 202 Intramuscular and intranasal preclinical study results ANIMAL HEALTH
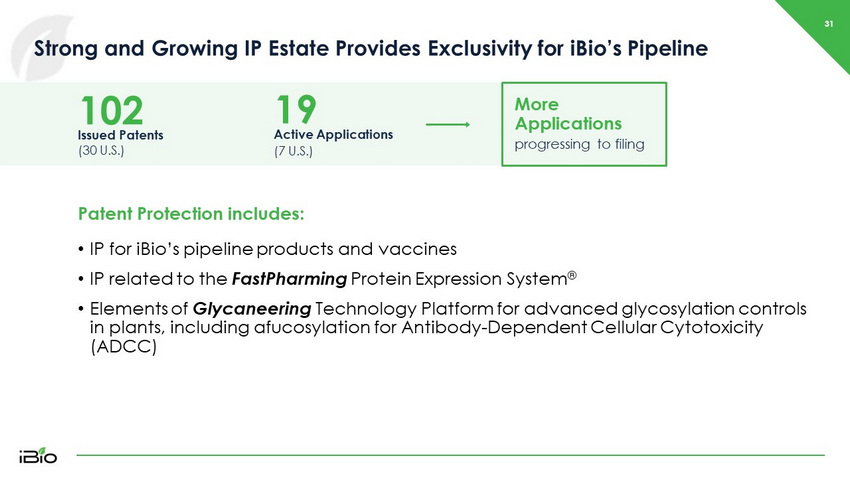
31 Strong and Growing IP Estate Provides Exclusivity for iBio’s Pipeline Issued Patents (30 U.S.) 102 Active Applications (7 U.S.) 19 More Applications progressing to filing Patent Protection includes: • IP for iBio’s pipeline products and vaccines • IP related to the FastPharming Protein Expression System ® • Elements of Glycaneering Technology Platform for advanced glycosylation controls in plants, including afucosylation for Antibody - Dependent Cellular Cytotoxicity (ADCC)

32 Financial Overview Publicly traded (NYSEA: IBIO) since January 2008 Approximately $103.9 million in cash and cash equivalents plus investments in debt securities as of 31 March 2021 Current cash provides a runway at least through March 2023 Approximately 216.0 million common shares and approximately 5.7 million options and restricted stock units to purchase shares of common stock outstanding as of 31 March 2021 No debt