As filed with the Securities and Exchange Commission on April 24, 2009
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
Under
THE SECURITIES ACT OF 1933
IBIOPHARMA, INC.
(Exact Name of Registrant as Specified in Its Charter)
|
Delaware |
2834 |
26-2797813 |
|
(State of Other Jurisdiction of |
(Primary Standard Industrial |
(I.R.S. Employer |
9 Innovation Way, Suite 100, Newark, Delaware 19711
(Address of Principal Executive Offices, including Zip Code)
Robert B. Kay
Chief Executive Officer
9 Innovation Way, Suite 100
Newark, Delaware 19711
(302) 355-0650
(Name, Address and Telephone Number of Agent for Service)
with copies to:
Andrew H. Abramowitz
Davis Wright Tremaine LLP
1633 Broadway
27th Floor
New York, New York 10019
(212) 489-8340 (fax)
Approximate date of commencement of proposed sale to the public: From time to time after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “accelerated filer,” “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer |_| Accelerated filer |_| Non-accelerated filer |_| Smaller reporting company |X|
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered |
Amount to be |
Proposed |
Proposed |
Amount of |
||||
|
|
||||||||
|
Common Stock, Par Value $0.001 |
2,345,752 |
$0.31 (2) |
$727,183.12 |
$40.58 |
||||
|
Common Stock, Par Value $0.001 (3) |
1,172,876 |
$3.20 (4) |
$3,753,203.20 |
$209.43 |
||||
|
Common Stock, Par Value $0.001 (5) |
1,172,876 |
$4.26 (4) |
$4,996,451.76 |
$278.80 |
||||
|
|
||||||||
(1) In addition to the shares set forth in the table, pursuant to Rule 416(a) under the Securities Act of 1933, as amended, this registration statement also covers an indeterminable number of shares of common stock that may be issuable as a result of stock splits, stock dividends and anti-dilution provisions.
(2) Estimated pursuant to Rule 457(c) solely for the purposes of calculating amount of the registration fee; computed, pursuant to Rule 457(c), upon the basis of the average of the high and low prices of the common stock as quoted on the OTC Bulletin Board on April 16, 2009.
(3) Represents shares of common stock issuable upon the exercise of warrants at an exercise price of $3.20 per share.
(4) Calculated pursuant to Rule 457(g) based upon the price at which the warrants may be exercised.
(5) Represents shares of common stock issuable upon the exercise of warrants at an exercise price of $4.26 per share.
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED APRIL 24, 2009
PROSPECTUS
4,691,504 Shares
iBioPharma, Inc.
COMMON STOCK
The selling stockholders listed in this prospectus are offering and selling from time to time up to 4,691,504 shares of common stock of iBioPharma, Inc. Of these shares, 2,345,752 are issuable upon the exercise of outstanding warrants. We will receive none of the proceeds from the sale, except upon exercise of the warrants.
The selling stockholders may sell the securities from time to time on any stock exchange or automated interdealer quotation system on which the securities are listed, in the over-the-counter market, in privately negotiated transactions or otherwise, at fixed prices that may be changed, at market prices prevailing at the time of sale, at prices related to the prevailing market prices or at prices otherwise negotiated.
Our common stock is quoted on the OTC Bulletin Board under the symbol “IBPM.OB”. On April 16, 2009, the high and low bid prices for shares of our common stock were $0.31 and $0.31 per share, respectively.
Our business and an investment in our common stock involve significant risks. See “Risk Factors” beginning on page 3.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2009.
|
TABLE OF CONTENTS |
|
|
Page |
|
|
SUMMARY |
1 |
|
RISK FACTORS |
3 |
|
FORWARD-LOOKING STATEMENTS |
12 |
|
USE OF PROCEEDS |
12 |
|
DIVIDEND POLICY |
12 |
|
SELLING STOCKHOLDERS |
12 |
|
PLAN OF DISTRIBUTION |
14 |
|
DESCRIPTION OF OUR CAPITAL STOCK |
16 |
|
SHARES ELIGIBLE FOR FUTURE SALE |
18 |
|
DESCRIPTION OF BUSINESS |
19 |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
30 |
|
DESCRIPTION OF PROPERTY |
43 |
|
SECURITIES OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
44 |
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
45 |
|
EXECUTIVE COMPENSATION |
48 |
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
50 |
|
LEGAL PROCEEDINGS |
53 |
|
MARKET FOR AND DIVIDENDS ON THE COMMON STOCK |
54 |
|
LEGAL MATTERS |
54 |
|
EXPERTS |
54 |
|
WHERE YOU CAN FIND MORE INFORMATION |
54 |
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different information. Neither we nor the selling stockholders are making an offer of these securities in any state where the offer is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus. In this prospectus, the “Company,” “iBioPharma”, “we,” “us” and “our” refer to iBioPharma, Inc.
This summary highlights selected information contained elsewhere in this prospectus and may not contain all the information that you need to consider in making your investment decision. Before making a decision to purchase our common stock, you should read the entire prospectus carefully, including the “Risk Factors” and “Forward-Looking Statements” sections and our consolidated financial statements and the notes to those financial statements.
Our Company
iBioPharma, Inc., a Delaware corporation (formerly InB:Biotechnologies, Inc., a New Jersey corporation), is a biopharmaceutical company focused on using and promoting the use of our proprietary plant-based technology platform (which we refer to herein as the platform or our platform) by which targeted proteins can be produced in plants for the development and manufacture of novel vaccines and therapeutics for use in humans and for certain veterinary applications.
Recent Developments
In August 2008, we were spun off as a stand-alone company from our former parent, Integrated BioPharma, Inc.
Shortly following the spin-off, we entered into a Securities Purchase Agreement with accredited investors pursuant to which such investors purchased an aggregate of 2,345,752 shares of our common stock at a purchase price of $2.13 per share, for gross proceeds of $5,000,000. As part of the private placement, each investor was issued two five-year warrants, each to purchase 50% of the number of shares of common stock such investor purchased in the private placement. One warrant has an exercise price of 150% of the per share purchase price of the common stock in the private placement, and the other warrant has an exercise price of 200% of the per share purchase price of the common stock in the private placement.
Also shortly following the spin-off, we entered into a Conversion Agreement with Integrated BioPharma, pursuant to which we:
|
· |
converted $2,700,000 of inter-company debt owed to Integrated BioPharma into 1,266,706 shares of our common stock; and |
|
· |
contributed an additional $5,177,097 of such debt into additional paid-in capital. |
Our Corporate Information
We are a Delaware corporation. Our principal executive/administrative offices are located at 9 Innovation Way, Suite 100, Newark, Delaware 19711, and our telephone number is (302) 355-0650. Our website address is http://www.ibiopharma.com. Information on or accessed through our website is not incorporated into this prospectus and is not a part of this prospectus. Our common stock is quoted on the OTC Bulletin Board under the symbol “IBPM.OB.”
The Offering
|
Common stock offered by selling stockholders |
4,691,504 shares, consisting of 2,345,752 outstanding shares owned by selling stockholders and 2,345,752 shares issuable upon the exercise of certain warrants held by the selling stockholders. |
|
Common stock outstanding before the offering |
23,357,519 shares. |
|
Common stock outstanding after the offering |
25,703,271 shares.(1) |
|
Proceeds to us |
We will not receive any of the proceeds from the sale of the shares of common stock because they are being offered by the selling stockholders. We are not offering any shares for sale under this prospectus. However, we will receive the proceeds from any exercise of the warrants, which would be used for general corporate and working capital purposes. |
(1) Assumes the exercise of warrants to purchase 2,345,752 shares held by selling stockholders.
RISK FACTORS
Our past experience may not be indicative of future performance, and as noted elsewhere in this prospectus, we have included forward-looking statements about our business, plans and prospects that are subject to change. Forward-looking statements are particularly located in, but not limited to, the sections “Description of Business” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In addition to the other risks or uncertainties contained in this prospectus, the following risks may affect our operating results, financial condition and cash flows. If any of these risks occur, either alone or in combination with other factors, our business, financial condition or operating results could be adversely affected. Moreover, readers should note this is not an exhaustive list of the risks we face; some risks are unknown or not quantifiable, and other risks that we currently perceive as immaterial may ultimately prove more significant than expected. Statements about plans, predictions or expectations should not be construed to be assurances of performance or promises to take a given course of action.
Risks Relating to our Business
Our plant-based technology platform has not previously been used by others to successfully develop products, and if we are not able to establish licenses of the platform, we may not generate sufficient license revenues to fulfill our business plan.
If we are unable to convince others to adopt the use of the platform in addition to or instead of other methods to produce vaccines and therapeutic proteins, we might not generate the revenues presently contemplated by our business plan to support our continuing operations.
Our product candidates are in the preclinical stage of development, and if we or our licensees are not able to successfully develop and commercialize them, we may not generate sufficient revenues to continue our business operations.
We have five internal product candidates and two additional categories--biodefense and developing world--made through the application of our technology platform, none of which has entered human clinical trials and for none of which an investigational new drug application (IND) has been filed with the FDA. Our success in establishing licenses to our platform will substantially depend on our ability to successfully complete clinical trials, obtain required regulatory approvals for our product candidates alone or with other persons. If the studies described above or any further studies fail, if we do not obtain required regulatory approvals, or if we fail to commercialize any of our product candidates alone or with licensees, we may be unable to generate sufficient revenues to attain profitability or continue our business operations, and our reputation in the industry and in the investment community would likely be significantly damaged, each of which would cause our stock price to decline and your holdings of our stock to lose most, if not all, of their value.
We will not be able to commercialize our product candidates if our preclinical studies do not produce successful results or our clinical trials do not demonstrate safety and efficacy in humans.
Preclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and has an uncertain outcome. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent our ability to commercialize our product candidates, including the following:
|
· |
Our preclinical or clinical trials may produce negative or inconclusive results, which may require us to conduct additional preclinical testing or clinical trials or to abandon projects that we expect to be promising. For example, we may obtain promising animal data about the immunogenicity of a vaccine candidate and then our human tests may result in no or inadequate immune responses. In addition, we may encounter unexpected safety concerns that would require further testing even if the vaccine candidate produced a very significant immune response in human subjects. |
|
· |
Initial clinical results may not be supported by further or more extensive clinical trials. For example, we may obtain data that suggest a desirable immune response from one of our vaccine candidates in a small human study, but then when tests are conducted on larger numbers of people, we may not see the same extent of immune response. If the immune response generated by a vaccine is too low, or occurs in too few treated individuals, then the vaccine will have no commercial value. |
|
· |
Enrollment in our clinical trials may be slower than we currently anticipate, resulting in significant delays. The cost of conducting a clinical trial increases as the time required to enroll adequate numbers of human subjects to obtain meaningful results increases. Enrollment in a clinical trial can be a slower-than-anticipated process because of competition from other clinical trials, because the study is not of interest to qualified subjects, or because the |
3
|
|
stringency of requirements for enrollment limits the number of people who are eligible to participate in the clinical trial. |
|
· |
We might have to suspend or terminate our clinical trials if the participating patients are being exposed to unacceptable health risks. Animal tests do not always adequately predict potential safety risks to human subjects. We will not know the risk of any candidate product until it is tested in human subjects, and if subjects experience adverse events during the clinical trial, the trial may have to be suspended and modified or terminated entirely. |
|
· |
Regulators or institutional review boards may suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements. |
|
· |
Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the product not commercially viable. |
|
· |
The effects of our product candidates may not be the desired effects or may include undesirable side effects. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing, or if the results of these trials or tests are not positive or are only modestly positive, we may be delayed in obtaining marketing approval for our product candidates, we may not be able to obtain marketing approval or we may obtain approval for indications that are not as broad as intended. Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether planned clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Significant clinical trial delays could allow our competitors to bring products to market before we do and impair our ability to commercialize our products or product candidates. Poor clinical trial results or delays may make it impossible to license a product or so reduce its attractiveness to a licensing partner that we will be unable to successfully commercialize a product.
Current economic conditions may cause a decline in business and consumer spending which could adversely affect our business and financial performance.
Our operating results are impacted by the health of the North American economies. Our business and financial performance, including collection of our accounts receivable, recoverability of assets including investments, may be adversely affected by current and future economic conditions, such as a reduction in the availability of credit, financial market volatility, recession, etc.
Additionally, we may experience difficulties in scaling our operations to react to economic pressures in the U.S.
We will need substantial additional funding to shepherd our product candidates through the clinical testing process and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our research and development expenses to increase in connection with our ongoing activities, particularly as the scope of the clinical trials that we are conducting expands. In addition, subject to regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We will need substantial additional funding and may be unable to raise capital when needed or may be unable to raise capital on attractive terms, which would force us to delay, reduce or eliminate our research and development programs or commercialization efforts.
We believe that our existing cash resources, along with our $5.0 million private placement of common stock that closed in August 2008, as described herein, and support from FhCMB collaborators, will be sufficient to meet our projected operating requirements only through the first calendar quarter of 2010. Our future funding requirements will depend on many factors, including:
|
· |
the scope and results of our clinical trials; |
|
· |
our ability to advance additional product candidates into development; |
|
· |
the success of our anticipated commercial agreements with pharmaceutical companies; |
|
· |
our ability to establish and maintain additional development agreements or other alternative arrangements; |
4
|
· |
the timing of, and the costs involved in, obtaining regulatory approvals; |
|
· |
the cost of manufacturing activities; |
|
· |
the cost of commercialization activities, including product marketing, sales and distribution; |
|
· |
the costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims and other patent-related costs, including, if necessary, litigation costs and the results of such litigation; and |
|
· |
potential acquisition or in-licensing of other products or technologies. |
We estimate we would need to raise additional funds of approximately $35 million over the next three years to operate our business if we were to independently fund a Phase 3 clinical trial of one of our product candidates. Our funding needs would likewise increase as we move additional product candidates through the clinical trial process.
If we are unsuccessful in raising additional capital or other alternative financing, we might have to defer or abandon part or all of our efforts to commercialize the intellectual property obtained from FhCMB and even cease operations.
Our product development and commercialization involve a number of uncertainties, and we may never generate sufficient revenues from the sale of potential products to become profitable; therefore, we may raise funds which may be dilutive of our shareholders in the future.
We have generated no significant revenues to date. To generate revenue and to achieve profitability, we must successfully develop licenses for our platform and/or clinically test, market and sell our potential products. Even if we generate revenue and successfully achieve profitability, we cannot predict the level of that profitability or whether it will be sustainable. We expect that our operating results will fluctuate from period to period as a result of differences in when we incur expenses and receive revenues from sales of our potential products, business arrangements and other sources. Some of these fluctuations may be significant.
Until we can generate a sufficient amount of license and/or product revenue, if ever, we expect to finance future cash needs through public or private equity offerings, debt financings and corporate product or technology development agreements and licensing arrangements. If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Debt financing, if available, may involve restrictive covenants. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to us or our stockholders. If we raise additional funds through development and licensing arrangements with third parties, it will be necessary to relinquish valuable rights to our technologies, research programs or product candidates or grant licenses on terms that may not be favorable to us.
Even if we or our potential licensees successfully complete clinical trials for our product candidates, there are no assurances that we will be able to submit, or obtain FDA approval of, a new drug application or biologics license application.
There can be no assurance that, if clinical trials for any of our product candidates are successfully completed, we will be able to submit a biologics license application (BLA), to the FDA or that any BLA we submit will be approved by the FDA in a timely manner, if at all. After completing clinical trials for a product candidate in humans, a dossier is prepared and submitted to the FDA as a BLA, and includes all preclinical and clinical trial data that clearly establish both short-term and long-term safety for a product candidate, and data that establishes the statistically significant efficacy of a product candidate, in order to allow the FDA to review such dossier and to consider a product candidate for approval for commercialization in the United States. If we are unable to submit a BLA with respect to any of our product candidates, or if any BLA we submit is not approved by the FDA, we will be unable to commercialize that product. The FDA can and does reject BLAs and requires additional clinical trials, even when product candidates perform well or achieve favorable results in large-scale Phase 3 clinical trials. If we fail to commercialize any of our product candidates, we may be unable to generate sufficient revenues to continue operations or attain profitability and our reputation in the industry and in the investment community would likely be damaged, each of which would cause our stock price to significantly decrease.
If commercialized, our product candidates may not be approved for sufficient governmental or third-party reimbursements, which would adversely affect our ability to market our product candidates.
In the United States and elsewhere, sales of pharmaceutical products depend in significant part on the availability of reimbursement to the consumer from third-party payers, such as government and private insurance plans. Since we currently have no commercial products, we have not had to face this issue yet; however, third-party payers are increasingly challenging
5
the prices charged for medical products and services. It will be time consuming and expensive for us to go through the process of seeking reimbursement from Medicaid, Medicare and private payers for any of our product candidates. Our products may not be considered cost effective, and coverage and reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis. The passage of the Medicare Prescription Drug and Modernization Act of 2003 imposes new requirements for the distribution and pricing of prescription drugs which may negatively affect the marketing of our potential products.
We face competition from many different sources, including pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions, and such competition may adversely affect our ability to generate revenue from our products.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, clinical trials, regulatory approvals and marketing approved products than we do. For example, large pharmaceutical companies are in the influenza vaccine business. Five injectable influenza vaccines are approved for use in the U.S. These include Afluria made by CSL Limited, Fluzone made by Sanofi-Pasteur, Fluarix made by GlaxoSmithKline, Flulaval made by ID Biomedical and distributed by GlaxoSmithKline, and Fluvirin made by Novartis. In addition, a nasally-administered influenza vaccine called FluMist is made by MedImmune. If we are successful in obtaining regulatory approval for our influenza vaccine candidate, these large companies would be our competitors.
Smaller or early stage companies may also prove to be significant competitors, particularly through business arrangements with large and established companies that may reduce the potential demand for access to our platform. For example, Novavax is conducting human clinical trials of vaccines for influenza and other infectious diseases using cell culture processes for manufacturing, and Medicago has announced preclinical experiments to produce influenza vaccines in green plants.
There are currently approved therapies for the diseases and conditions addressed by our vaccine and antibody candidates that are undergoing clinical trials and for the diseases and conditions that are subjects of our preclinical development program. Our commercial opportunities will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that we may develop. For example, the drugs oseltamivir, amantadine, and zanamivir are used to treat certain influenza infections, and Merck’s vaccine to prevent HPV infection has been approved by the FDA with a similar vaccine developed by GlaxoSmithKline in late-stage development. There are also a number of companies working to develop new drugs and other therapies for diseases of commercial interest to us that are undergoing various stages of testing including clinical trials. The key competitive factors affecting the success of all of our product candidates are likely to be their efficacy, safety profile, price and convenience.
Finally, these third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
We will depend significantly on arrangements with third parties to develop and commercialize our product candidates.
A key element of our business strategy and our thinly-staffed employment structure is to establish arrangements with licensees, particularly leading pharmaceutical and biotechnology companies, to develop and commercialize product candidates. We and FhCMB currently are working within our business structure, which includes non-commercial arrangements as described above, to apply further our plant-based platform technology. Delays, withdrawals or other adverse changes to the current participants in our business structure might adversely affect our ability to develop and commercialize our product candidates.
We expect to rely upon our future business arrangements for support in advancing certain of our drug candidates and intend to rely on additional work under current and future arrangements during our efforts to commercialize our product candidates. Our contractors may be conducting multiple product development efforts within the same disease areas that are the subjects of their agreements with us. Our agreements might not preclude them from pursuing development efforts using a different approach from that which is the subject of our agreement with them. Any of our drug candidates, therefore, may be subject to competition with a drug candidate under development by a contractor.
The success of our business arrangements will depend heavily on the efforts and activities of the organizations which are party to these arrangements. Our future contractual arrangements may provide significant discretion in determining the efforts and resources available to these programs. The risks that we face in connection with these arrangements, and that we anticipate
|
· |
Future agreements may be for fixed terms and subject to termination under various circumstances, including, in some cases, on short notice without cause. |
6
|
· |
Our future licensees may develop and commercialize, either alone or with others, products and services that are similar to or competitive with the products that are the subject of the agreement with us. |
|
· |
Our future licensees may underfund or not commit sufficient resources to the testing, marketing, distribution or other development of our products. |
|
· |
Our future licensees may not properly maintain or defend our intellectual property rights, or they may utilize our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential liability. |
|
· |
Our future licensees may change the focus of their development and commercialization efforts. Pharmaceutical and biotechnology companies historically have re-evaluated their priorities from time to time, including following mergers and consolidations, which have been common in recent years in these industries. The ability of our product candidates and products to reach their potential could be limited if our licensees or customers decrease or fail to increase spending relating to such products. |
Business arrangements with pharmaceutical companies and other third parties often are terminated or allowed to expire by the other party. Such terminations or expirations would adversely affect us financially and could harm our business reputation.
We may not be successful in establishing additional arrangements with third parties, which could adversely affect our ability to discover, develop and commercialize products.
We engaged FhCMB to perform research and development activities to apply our platform technology to create product candidates. We currently do not have other similar agreements with third parties. If we are able to obtain such agreements, however, these arrangements may not be scientifically or commercially successful. If we are unable to reach new agreements with suitable third parties, we may fail to meet our business objectives for the affected product or program. We face significant competition in seeking appropriate companies with which to create additional similar business structures. Moreover, these arrangements are complex to negotiate and time-consuming to document. We may not be successful in our efforts to establish additional alternative arrangements. The terms of any additional arrangements that we establish may not be favorable to us. Moreover, these arrangements may not be successful.
If third parties on whom we will rely for clinical trials do not perform as contractually required or as we expect, we may not be able to obtain regulatory approval for or commercialize our product candidates, and our business may suffer.
We do not have the ability to independently conduct the clinical trials required to obtain regulatory approval for our products. We have not yet contracted with any third parties to conduct our clinical trials. We will depend on independent clinical investigators, contract research organizations and other third party service providers to conduct the clinical trials of our product candidates and expect to continue to do so. We will rely heavily on these parties for successful execution of our clinical trials but will not control many aspects of their activities. For example, the investigators may not be our employees. However, we will be responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Third parties may not complete activities on schedule, or may not conduct our clinical trials in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations could delay or prevent the development, approval and commercialization of our product candidates.
We face substantial uncertainty in our ability to protect our patents and proprietary technology.
Our ability to commercialize our products will depend, in part, on our or our licensors’ ability to obtain patents, to enforce those patents and preserve trade secrets, and to operate without infringing on the proprietary rights of others.
The patent positions of biopharmaceutical companies like us are highly uncertain and involve complex legal and factual questions. To date, we currently hold one issued U.S. patent for inducing gene silencing in plants that expires on July 25, 2022 and one U.S. patent application describing systems for expression of vaccine antigens in plants for which we have received a notice of allowance. We have an additional 19 U.S. applications pending. We have also applied for patents in numerous foreign countries, including Europe, Canada, Australia, China, India, Brazil, Japan, Hong Kong and New Zealand for the intellectual property developed by FhCMB. We currently have 34 pending foreign patent applications. There can be no assurance that:
|
· |
patent applications owned by or licensed to us will result in issued patents; |
|
· |
patent protection will be secured for any particular technology; |
|
· |
any patents that have been or may be issued to us will be valid or enforceable; |
7
|
· |
any patents will provide meaningful protection to us; |
|
· |
others will not be able to design around the patents; or |
|
· |
our patents will provide a competitive advantage or have commercial application. |
The failure to obtain and maintain adequate patent protection would have a material adverse effect on us and may adversely affect our ability to enter into, or affect the terms of, any arrangement for the marketing of any product. Please see “Description of Our Business – Intellectual Property” for more information.
We cannot assure you that our patents will not be challenged by others.
There can be no assurance that patents owned by or licensed to us will not be challenged by others. We currently hold one issued U.S. patent for methods of inducing gene silencing in plants and one U.S. patent application for which we have received a notice of allowance, describing systems for expression of vaccine antigens in plants. Please see “Description of Our Business – Intellectual Property” for more information on our current patents and patent applications. We could incur substantial costs in proceedings, including interference proceedings before the United States Patent and Trademark Office and comparable proceedings before similar agencies in other countries in connection with any claims that may arise in the future. These proceedings could result in adverse decisions about the patentability of our or our licensors’ inventions and products, as well as about the enforceability, validity or scope of protection afforded by the patents. Any adverse decisions about the patentability of our product candidates could cause us to either lose rights to develop and commercialize our product candidates or to license such rights at substantial cost to us. In addition, even if we were successful in such proceedings, the cost and delay of such proceedings would most likely have a material adverse effect on our business.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information, may not adequately protect our intellectual property, and will not prevent third parties from independently discovering technology similar to or in competition with our intellectual property.
We rely on trade secrets and other unpatented proprietary information in our product development activities. To the extent we rely on trade secrets and unpatented know-how to maintain our competitive technological position, there can be no assurance that others may not independently develop the same or similar technologies. We seek to protect trade secrets and proprietary knowledge, in part, through confidentiality agreements with our employees, consultants, advisors, collaborators and contractors. Nevertheless, these agreements may not effectively prevent disclosure of our confidential information and may not provide us with an adequate remedy in the event of unauthorized disclosure of such information. If our employees, scientific consultants, advisors, collaborators or contractors develop inventions or processes independently that may be applicable to our technologies, product candidates or products, disputes may arise about ownership of proprietary rights to those inventions and processes. Such inventions and processes will not necessarily become our property, but may remain the property of those persons or their employers. Protracted and costly litigation could be necessary to enforce and determine the scope of our proprietary rights. If we fail to obtain or maintain trade secret protection for any reason, the competition we face could increase, reducing our potential revenues and adversely affecting our ability to attain or maintain profitability.
If we infringe or are alleged to infringe intellectual property rights of third parties, it will adversely affect our business.
Our research, development and commercialization activities, as well as any products candidates or products resulting from these activities, may infringe or be claimed to infringe patents or patent applications under which we do not hold licenses or other rights. Third parties may own or control these patents and patent applications in the United States and abroad. These third parties could bring claims against us or our customers, collaborators or licensees that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
As a result of patent infringement claims, or in order to avoid potential claims, we or our customers, collaborators or licensees may choose to seek, or be required to seek, a license from the third party and would most likely be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we or our customers, collaborators or licensees were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we or our customers, collaborators or licensees are unable to enter into licenses on acceptable terms. This could harm our business significantly.
8
There have been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference proceedings declared by the United States Patent and Trademark Office and opposition proceedings in the European Patent Office, regarding intellectual property rights with respect to our products and technology. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
There is a substantial risk of product liability claims in our business. If we are unable to obtain sufficient insurance, a product liability claim against us could adversely affect our business.
Clinical trial and product liability insurance is volatile and may become increasingly expensive. As a result, we may be unable to obtain sufficient insurance or increase our existing coverage at a reasonable cost to protect us against losses that could have a material adverse effect on our business. An individual may bring a product liability claim against us if one of our products or product candidates causes, or is claimed to have caused, an injury or is found to be unsuitable for consumer use. Any product liability claim brought against us, with or without merit, could result in:
|
· |
liabilities that substantially exceed our product liability insurance, which we would then be required to pay from other sources, if available; |
|
· |
an increase of our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable terms, or at all; |
|
· |
withdrawal of clinical trial volunteers or patients; |
|
· |
damage to our reputation and the reputation of our products, resulting in lower sales of any future commercialized product which we may have; |
|
· |
regulatory investigations that could require costly recalls or product modifications; |
|
· |
litigation costs; |
|
· |
the diversion of management’s attention from managing our business. |
Our inability to obtain adequate insurance coverage at an acceptable cost could prevent or inhibit the commercialization of our products. If third parties were to bring a successful product liability claim or series of claims against us for uninsured liabilities or in excess of insured liability limits, our business, financial condition and results of operations could be materially harmed.
Risks Relating to Our Relationship with and Spin-Off from Integrated BioPharma
Our business could suffer if our systems and infrastructure are inadequate or we cannot replace the other benefits previously provided by Integrated BioPharma.
Since our inception, we have relied on Integrated BioPharma for various services which we have only recently developed for ourselves, including:
|
· |
legal; |
|
· |
treasury; |
|
· |
tax; |
|
· |
employee benefits; |
|
· |
insurance; |
|
· |
investor relations; and |
9
|
· |
executive oversight and other services. |
As of August 18, 2008, following the distribution, we are operating as a separate publicly traded company. We have developed and implemented systems and infrastructure to support our current and future business, and our responsibilities as a public company. These systems and infrastructure may be inadequate, however, and we may be required to develop or otherwise acquire other systems and infrastructure, or to obtain certain corporate services from Integrated BioPharma to support our current and future business such as legal, strategic financial planning, tax and SEC reporting services.
As of August 18, 2008, subsequent to the distribution from Integrated BioPharma, we are not able to obtain financing from Integrated BioPharma.
Our plans to expand our business and to continue to improve our products may require funds in excess of our cash flow and will require us to seek financing from third parties. In the past, Integrated BioPharma has provided capital for our general corporate purposes, and we used cash provided by Integrated BioPharma to fund our operations. As of August 18, 2008, subsequent to the distribution, however, Integrated BioPharma is not providing funds to finance our operations. Without the opportunity to obtain financing from Integrated BioPharma, we will need to obtain additional financing from banks, or through public offerings or private placements of debt or equity securities, strategic relationships or other arrangements. We cannot give assurances at this time that we will be able to obtain such funding. In addition, the terms, interest rates, costs and fees of new credit facilities may not be as favorable as those historically enjoyed with Integrated BioPharma. For example, Integrated BioPharma did not charge us with any fees or costs for the intercompany borrowing, nor were there any covenants regarding financial ratios or prohibition on certain transactions in the loan arrangement with Integrated BioPharma. Our inability to obtain financing on favorable terms could restrict our operations and reduce our profitability. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources.”
The agreements we entered into with Integrated BioPharma in connection with the distribution could restrict our operations.
In connection with the distribution, we and Integrated BioPharma entered into a number of agreements that govern our spin-off from Integrated BioPharma and our future relationship. Each of these agreements were entered into in the context of our relationship to Integrated BioPharma as a subsidiary and our spin-off from Integrated BioPharma and, accordingly, the terms and provisions of these agreements may be less favorable to us than terms and provisions we could have obtained in arm’s-length negotiations with unaffiliated third parties. These agreements commit us to take actions, observe commitments and accept terms and conditions that are or may be advantageous to Integrated BioPharma but are or may be disadvantageous to us. The terms of these agreements include obligations and restrictive provisions, including, but not limited to:
|
· |
an agreement to indemnify Integrated BioPharma, its affiliates, and each of their respective directors, officers, employees, agents and representatives from certain liabilities arising out of any litigation we are involved in and all liabilities that arise from our breach of, or performance under, the agreements we are entering into with Integrated BioPharma in connection with the distribution and for any of our liabilities; and |
|
· |
an agreement with regard to tax matters between ourselves and Integrated BioPharma which restricts our ability to engage in certain strategic or capital raising transactions. |
Risks Relating to our Common Stock
Our future results may vary significantly in the future which may adversely affect the price of our common stock.
It is possible that our quarterly revenues and operating results may vary significantly in the future and that period-to-period comparisons of our revenues and operating results are not necessarily meaningful indicators of the future. You should not rely on the results of one quarter as an indication of our future performance. It is also possible that in some future quarters, our revenues and operating results will fall below our expectations or the expectations of market analysts and investors. If we do not meet these expectations, the price of our common stock may decline significantly.
Our common stock is considered “a penny stock” and may be difficult to sell.
The SEC has adopted regulations which generally define “penny stock” to be an equity security that has a market price of less than $5.00 per share or an exercise price of less than $5.00 per share, subject to specific exemptions. As the market price of our common stock has been less than $5.00 per share, our common stock is considered a “penny stock” according to SEC rules. This designation requires any broker or dealer selling these securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine that the purchaser is reasonably suitable to purchase
10
the securities. These rules may restrict the ability of brokers or dealers to sell our common stock and may affect the ability of investors to sell their shares. In addition, since our common stock is currently traded on the NASDAQ’s OTC Bulletin Board, investors may find it difficult to obtain accurate quotations for our common stock and may experience a lack of buyers to purchase such stock or a lack of market makers to support the stock price.
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable.
Provisions of our certificate of incorporation, bylaws and provisions of applicable Delaware law may discourage, delay or prevent a merger or other change in control that a stockholder may consider favorable. Pursuant to our certificate of incorporation, our board of directors may issue additional shares of common or preferred stock. Any additional issuance of common stock could have the effect of impeding or discouraging the acquisition of control of us by means of a merger, tender offer, proxy contest or otherwise, including a transaction in which our stockholders would receive a premium over the market price for their shares, and thereby protects the continuity of our management. Specifically, if in the due exercise of his/her or its fiduciary obligations, the board of directors were to determine that a takeover proposal was not in our best interest, shares could be issued by our board of directors without stockholder approval in one or more transactions that might prevent or render more difficult or costly the completion of the takeover by:
|
· |
diluting the voting or other rights of the proposed acquirer or insurgent stockholder group, |
|
· |
putting a substantial voting block in institutional or other hands that might undertake to support the incumbent board of directors, or |
|
· |
effecting an acquisition that might complicate or preclude the takeover. |
Our certificate of incorporation also allows our board of directors to fix the number of directors in the by-laws. Cumulative voting in the election of directors is specifically denied in our certificate of incorporation. The effect of these provisions may be to delay or prevent a tender offer or takeover attempt that a stockholder may determine to be in his, her or its best interest, including attempts that might result in a premium over the market price for the shares held by the stockholders.
We also are subject to Section 203 of the Delaware General Corporation Law. In general, these provisions prohibit a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an interested stockholder, unless the transaction in which the person became an interested stockholder is approved in a manner presented in Section 203 of the Delaware General Corporation Law. Generally, a “business combination” is defined to include mergers, asset sales and other transactions resulting in financial benefit to a stockholder. In general, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years, did own, 15% or more of a corporation’s voting stock. This statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire us.
We do not anticipate paying cash dividends for the foreseeable future, and therefore investors should not buy our stock if they wish to receive cash dividends.
We have never declared or paid any cash dividends or distributions on our capital stock. We currently intend to retain our future earnings to support operations and to finance expansion and therefore we do not anticipate paying any cash dividends on our common stock in the foreseeable future.
A significant number of our shares will be eligible for sale and their sale or potential sale may depress the market price of our common stock.
Sales of a significant number of shares of our common stock in the public market could harm the market price of our common stock. This prospectus covers 4,691,504 shares of our common stock, which represents approximately 20% of our currently outstanding shares of our common stock. As additional shares of our common stock become available for resale in the public market pursuant to this offering, and otherwise, the supply of our common stock will increase, which could decrease its price. Some or all of the shares of common stock may be offered from time to time in the open market pursuant to Rule 144, and these sales may have a depressive effect on the market for our shares of common stock. Subject to certain restrictions, a person who has held restricted shares for a period of six months may sell common stock into the market.
11
This prospectus contains forward-looking statements that involve risks and uncertainties. These forward-looking statements are not historical facts but rather are plans and predictions based on current expectations, estimates and projections about our industry, our beliefs and assumptions. We use words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate” and variations of these words and similar expressions to identify forward-looking statements. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and other factors, some of which are beyond our control, are difficult to predict and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. These risks and uncertainties include those described in the section above entitled “Risk Factors.” You should not place undue reliance on these forward-looking statements, which reflect our view only as of the date of this prospectus.
We will not receive any of the proceeds from the sale of the shares of common stock because they are being offered by the selling stockholders. We are not offering any shares for sale under this prospectus. However, we will receive the proceeds from any exercise of the warrants, which would be used for general corporate and working capital purposes.
DIVIDEND POLICY
We have never declared or paid any cash dividends or distributions on our capital stock. We currently intend to retain our future earnings to support operations and to finance expansion and therefore we do not anticipate paying any cash dividends on our common stock in the foreseeable future.
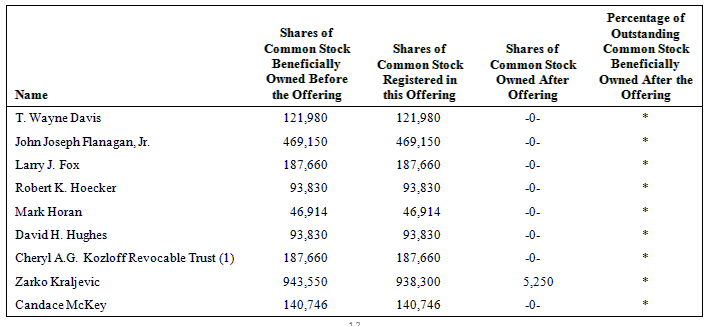
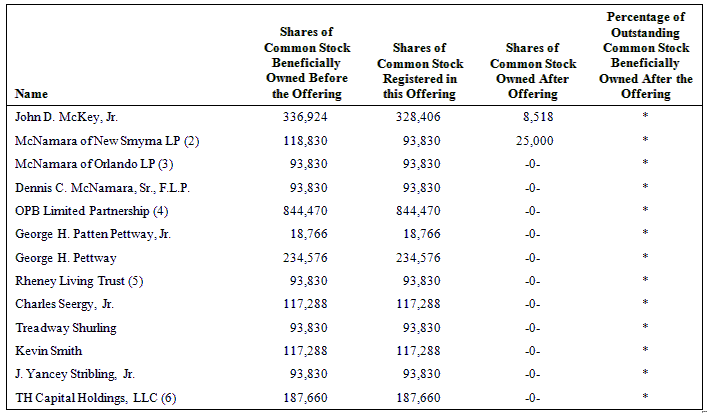
The following table sets forth the name of each of the selling stockholders, the number of shares beneficially owned by each of the selling stockholders as of April 15, 2009, the number of shares that may be offered under this prospectus and the number of shares of our common stock owned by each of the selling stockholders after the offering is completed.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting and investment power with respect to securities. To our knowledge, except as set forth in the footnotes to this table and subject to applicable community property laws, each person named in this table has sole voting and investment power with respect to the shares shown as beneficially owned by him or her.
As of April 15, 2009, 23,357,519 shares of our common stock were outstanding. In computing the number of shares beneficially owned by a person and the percentage of ownership of that person, shares of common stock issuable upon the exercise of warrants and options that are currently exercisable or exercisable within 60 days of April 15, 2009, are deemed to be outstanding and beneficially owned by the person holding the options, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.

12
____________________________
* less than 1%
(1) Cheryl A.G. Kozloff is the trustee of Cheryl A.G. Kozloff Revocable Trust, which is the registered holder of the shares of common stock. Cheryl A.G. Kozloff, as trustee of Cheryl A.G. Kozloff Revocable Trust, has voting and disposition power over the shares owned by Cheryl A.G. Kozloff Revocable Trust.
(2) Dennis C. McNamara, Jr. is the general partner of McNamara of New Smyrna LP, which is the registered holder of the shares of common stock. Dennis C. McNamara, Jr., as general partner of McNamara of New Smyrna LP, has voting and disposition power over the shares owned by McNamara of New Smyrna LP.
(3) Hal McNamara is the general partner of McNamara of Orlando LP, which is the registered holder of the shares of common stock. Hal McNamara, as general partner of McNamara of Orlando LP, has voting and disposition power over the shares owned by McNamara of Orlando LP.
(4) Bradley Hoecker is the general partner of OPB Limited Partnership, which is the registered holder of the shares of common stock. Bradley Hoecker, as general partner of OPB Limited Partnership, has voting and disposition power over the shares owned by OPB Limited Partnership.
(5) Samuel Clarke Rheney, Jr. is the trustee of Rheney Living Trust, which is the registered holder of the shares of common stock. Samuel Clarke Rheney, Jr., as trustee of Rheney Living Trust, has voting and disposition power over the shares owned by Rheney Living Trust.
(6) Michael Cirillo is the vice president of TH Capital Holdings, LLC, which is the registered holder of the shares of common stock. Michael Cirillo, as vice president of TH Capital Holdings, LLC, has voting and disposition power over the shares owned by TH Capital Holdings, LLC.
We are registering the shares of our common stock covered by this prospectus for the selling stockholders. As used in this prospectus, “selling stockholders” includes the donees, transferees or others who may later hold the selling stockholders’ interests. The selling stockholders will act independently of us in making decisions with respect to the timing, manner and size of each sale. The selling stockholders may, from time to time, sell all or a portion of their shares of common stock on the OTC Bulletin Board or on any national securities exchange or automated inter-dealer quotation system on which our common stock may be listed or traded, in negotiated transactions or otherwise, at prices then prevailing or related to the current market price or at negotiated prices. One or more underwriters on a firm commitment or best efforts basis may sell the shares of common stock directly or through brokers or dealers or in a distribution. The methods by which the shares of common stock may be sold include:
|
· |
ordinary brokerage transactions and transactions in which the broker/dealer solicits purchasers; |
|
· |
block trades (which may involve crosses) in which the broker/dealer will attempt to sell the shares as agent but may position and resell a portion of the block, as principal, to facilitate the transaction; |
|
· |
purchases by a broker/dealer, as principal, and resale by the broker/dealer for its account; |
|
· |
an exchange distribution in accordance with the rules of the applicable exchange; |
|
· |
privately negotiated transactions; |
|
· |
put or call options transactions; |
|
· |
settlement of short sales; |
|
· |
broker/dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; |
|
· |
a combination of any such methods of sale; and |
|
· |
any other method permitted by applicable law. |
In addition, any of the shares of common stock that qualify for sale pursuant to Rule 144 promulgated under the Securities Act of 1933 may be sold in transactions complying with that Rule, rather than pursuant to this prospectus.
For sales to or through broker-dealers, these broker-dealers may receive compensation in the form of discounts, concessions or commissions from the selling stockholders or the purchasers of the shares, or both. We have advised the selling stockholders that the anti-manipulative provisions of Regulation M under the Securities Exchange Act of 1934 may apply to their sales in the market and have informed them that they must deliver copies of this prospectus. We are not aware, as of the date of this prospectus, of any agreements between any of the selling stockholders and any broker-dealers with respect to the sale of the shares of common stock covered by this prospectus.
The selling stockholders and any broker-dealers or agents participating in the distribution of our shares may be deemed to be “underwriters” within the meaning of the Securities Act of 1933, and any commissions received by any broker-dealer or agent and profit on any resale of shares of common stock may be deemed to be underwriting commissions under the Securities Act of 1933. The commissions received by a broker-dealer or agent may be in excess of customary compensation. If a selling stockholder is deemed to be an “underwriter,” the selling stockholder may have liability for the accuracy of the contents of this prospectus under the Securities Act of 1933.
At a time a particular offer of shares is made by a selling stockholder, a prospectus supplement, if required, will be distributed that will set forth the names of any underwriters, dealers or agents and any discounts, commissions and other terms constituting compensation from the selling stockholders and any other required information.
The selling stockholders may from time to time pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the donees, pledgees or secured parties may offer and sell the shares of common stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act of 1933 amending the list of selling stockholders to include the donee, pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
14
The selling stockholders will be subject to applicable provisions of the Securities Exchange Act of 1934 and its rules and regulations, including without limitation, Rule 102 under Regulation M. These provisions may limit the timing of purchases and sales of our common stock by the selling stockholders. Rule 102 under Regulation M provides, with limited exceptions, that it is unlawful for the selling stockholders or their affiliated purchasers to, directly or indirectly, bid for or
purchase or attempt to induce any person to bid for or purchase, for an account in which the selling stockholders or affiliated purchasers have a beneficial interest in any securities that are the subject of the distribution during the applicable restricted period under Regulation M. All of the above may affect the marketability of our common stock.
Pursuant to applicable rules and regulations under the Securities Exchange Act of 1934, any person engaged in the distribution of the securities offered under this prospectus may not simultaneously engage in market activities for the shares of common stock for a period of five business days prior to the commencement of such distribution.
We will receive none of the proceeds from the sale of the shares of common stock by the selling stockholders, except upon exercise of warrants presently outstanding.
We will pay all costs and expenses incurred in connection with the registration under the Securities Act of 1933 of the shares of common stock offered by the selling stockholders, including all registration and filing fees, listing fees, printing expenses, and our legal and accounting fees. We estimate that these fees and expenses will total approximately $41,000. The selling stockholders will pay all of their own brokerage fees and commissions, if any, incurred in connection with the sale of their shares of common stock. In addition, we have agreed to indemnify the selling stockholders against certain liabilities, including liabilities under the Securities Act of 1933. We cannot assure you, however, that any of the selling stockholders will sell any or all of the shares of common stock they may offer.
15
DESCRIPTION OF OUR CAPITAL STOCK
Our authorized capital stock consists of 50,000,000 shares of common stock, par value $0.001 per share, and 1,000,000 shares of preferred stock. As of April 15, 2009, 23,357,519 shares of our common stock were issued and outstanding, and no shares of our preferred stock were outstanding.
Common Stock
The holders of our common stock are entitled to one vote for each share on all matters voted on by stockholders, including elections of directors, and, except as otherwise required by law or provided in any resolution adopted by our Board with respect to any series of preferred stock, the holders of such shares possess all voting power. Subject to any preferential rights of any outstanding series of our preferred stock created by our Board from time to time, the holders of common stock are entitled to such dividends as may be declared from time to time by our Board from funds available therefor and upon liquidation are entitled to receive pro rata the value of all assets available for distribution to such holders.
The holders of our common stock have no preemptive rights. The rights, preferences and privileges of holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future. All outstanding shares of our common stock are fully paid and non-assessable.
Preferred Stock
Under our amended and restated Certificate of Incorporation, the Board of Directors has the authority, without further action by stockholders, to issue up to 1,000,000 shares of preferred stock. The board may issue preferred stock in one or more series and may determine the rights, preferences, privileges, qualifications and restrictions granted to or imposed upon the preferred stock, including dividend rights, conversion rights, voting rights, rights and terms of redemption, liquidation preferences and sinking fund terms, any or all of which may be greater than the rights of the common stock. The issuance of preferred stock could adversely affect the voting power of holders of common stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation. The issuance of preferred stock could also have the effect of decreasing the market price of the common stock and could delay, deter or prevent a change in control of our company. We have no present plans to issue any shares of preferred stock.
Anti-Takeover Provisions
In addition to the agreement we have entered into with Integrated BioPharma that until August 2010 requires us to obtain the consent of the Integrated BioPharma Board of Directors to any transaction or issuance of our common stock that could result in a change in control of iBioPharma, various provisions contained in our amended and restated Certificate of Incorporation and By-laws could delay or discourage some transactions involving an actual or potential change in control of us or our management. These provisions may limit the ability of stockholders to remove current management or approve transactions that stockholders may deem to be in their best interests and could adversely affect the price of our common stock. These provisions contained in our amended and restated Certificate of Incorporation and By-laws could delay or discourage some transactions involving an actual or potential change in control of us or our management and may limit the ability of stockholders to remove current management or approve transactions that stockholders may deem to be in their best interests and could adversely affect the price of our common stock. These provisions:
|
· |
authorize our board of directors to establish one or more series of undesignated preferred stock, the terms of which can be determined by the board of directors at the time of issuance; |
|
· |
divide our board of directors into three classes of directors, with each class serving a staggered three-year term. As the classification of the board of directors generally increases the difficulty of replacing a majority of the directors, it may tend to discourage a third party from making a tender offer or otherwise attempting to obtain control of us and may maintain the composition of the board of directors; |
|
· |
prohibit cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient percentage of a class of shares may be able to ensure the election of one or more directors; |
|
· |
establish advance notice requirements for submitting nominations for election to the board of directors and for proposing matters that can be acted upon by stockholders at a meeting; |
16
|
· |
allow our directors, not our stockholders, to fill vacancies on our board of directors; and |
|
· |
provide that the authorized number of directors may be changed only by resolution of the board of directors. |
Continental Stock Transfer & Trust Company is the transfer agent and registrar for our common stock.
Our common stock is quoted on the OTC Bulletin Board under the symbol “IBPM.OB.”
Shares Covered by this Prospectus
All of the 4,691,504 shares of common stock being registered in this offering may be sold without restriction under the Securities Act, so long as the registration statement of which this prospectus is a part is, and remains, effective.
Rule 144
The SEC has recently adopted amendments to Rule 144 which became effective on February 15, 2008. Under these amendments, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the three months preceding, a sale and (ii) there is available current public information about us.
Persons who have beneficially owned restricted shares of our common stock for at least six months but who are our affiliates at the time of, or at any time during the three months preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of either of the following:
|
· |
1% of the total number of securities of the same class then outstanding; or |
|
· |
the average weekly trading volume of such securities during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale; |
|
· |
provided, in each case, that there is available current public information about us; |
|
· |
Such sales by affiliates must also comply with the manner of sale and notice provisions of Rule 144. |
We believe that 2,345,752 of our outstanding shares may currently be sold in reliance on Rule 144.
18
iBioPharma, Inc., a Delaware corporation (formerly InB:Biotechnologies, Inc., a New Jersey corporation) is a biopharmaceutical company focused on using and promoting the use of our proprietary plant-based technology platform (which we refer to herein as the platform or our platform) by which targeted proteins can be produced in plants for the development and manufacture of novel vaccines and therapeutics for use in humans and for certain veterinary applications.
This platform was invented and developed by Fraunhofer USA Center for Molecular Biotechnology (“FhCMB”), a not-for-profit translational research institution. In January 2004, we acquired the platform from FhCMB together with FhCMB’s commitment for the maintenance and support necessary to further protect the intellectual property comprising the platform, including filing and prosecuting patent applications, providing scientific support for patent counsel’s activities on our behalf and otherwise to maintain in force and good standing our intellectual property rights.
Our business model contemplates that, in addition to using our platform to create and advance our own product candidates, we will license the platform to, or enter into joint ventures or other business arrangements with, other parties (collectively, we refer to these third parties as licensees) who wish to use the platform for the development and/or production of their own product candidates. In order to attract appropriate licensees and increase the value of our share of such contractual arrangements, we engaged FhCMB in October 2004 to perform research and development activities to apply the platform to create our first product candidate. We selected a plant-based flu vaccine for human use as the product candidate to exemplify the value of the platform particularly for products that require rapid, highly-scalable and economic production. Performance of this first research agreement, which required us to make payments to FhCMB against the achievement of stated research milestones, has progressed through preclinical challenge studies in the ferret model. We thereafter suspended further work under that research agreement in favor of having pandemic flu vaccine using our platform become the first vaccine candidate to seek FDA permission for clinical trials. The pandemic flu vaccine candidate trials are expected to be financed by a grant from the Bill and Melinda Gates Foundation to FhCMB.
In addition, in 2006, we engaged FhCMB to create a prototype production module for products made through the use of the platform. The purpose for this engagement was to demonstrate the ease and economy with which platform-based products could be manufactured, again in order to attract potential licensees and increase the value of our share of business arrangements. The prototype design, which encompasses the entire production process from the seeding through pre-infiltration plant growth, infiltration with agrobacteria, harvesting of plant tissue and purification of target proteins, was completed in May 2008. Fabricated equipment for the prototype is scheduled to be delivered to FhCMB by June 2009. Equipment in the facility is scheduled to be commissioned and the facility validated for current Good Manufacturing Practices (called cGMP) production in the third quarter of 2009. The facility will then be used for pilot scale production of protein targets for clinical trials of product candidates which use our platform technology.
In addition to our direct funding of FhCMB’s application of the platform technology to our human flu vaccine product candidate, we have established non-commercial arrangements among us, certain government entities, a non-governmental organization (which we refer to herein as a NGO) and FhCMB, pursuant to which we grant non-commercial rights to use our platform for the development and production by FhCMB of product candidates selected by the government entities and NGO, in consideration for grants by the government entities and NGO directly to FhCMB to fund such research and development.
Through (i) iBioPharma/FhCMB contracts and (ii) the non-commercial arrangements described above, we retain ownership of the intellectual property and exclusive commercial rights in the fields of human health and veterinary influenza applications of the intellectual property. We license or otherwise grant use rights (a) to government and NGO entities for not-for-profit applications of the intellectual property for the development or application of which they granted or were granted funding, and (b) to FhCMB for research purposes and applications in other fields. This business structure enables us to obtain commercial rights to various applications of our platform technology funded by government entities and NGOs. It also helps us demonstrate the validity and apparent value of the platform to parties to whom we will offer licenses or other business opportunities. Our use of FhCMB to perform research and development work allows us to develop our product candidates, and thereby promote the value of our platform for licensing and product development purposes, without bearing the full risk and expense of establishing and maintaining our own research and development staff and facilities.
Using this business structure, we have applied our platform technology to create a pipeline of proprietary product candidates which we can offer to licensees, including vaccine and therapeutic candidates against seasonal and pandemic influenza, human papilloma virus (HPV), and other pathogens of public health significance. All of our product candidates are in the preclinical development stage, and to date, none of our product candidates has been approved by the FDA.
19
We have exclusive control over and the rights to ownership of the intellectual property related to human health and veterinary influenza applications of the plant-based technology developed by FhCMB. Current development projects include expansion of production capabilities, conducting proof-of-principle preclinical studies and planning clinical studies of proprietary influenza and HPV vaccines and antibodies for potential treatment and diagnosis of influenza infections.
Biotech drugs are proteins such as antibodies, blood proteins and enzymes. Many biotech drugs have been on the market long enough for patents on them to expire. Emerging opportunities for biosimilars (also known as biogenerics or follow-on biologics) creates potential for our platform technology to be used by potential licensees to enter the market due to what we expect to be an economical production system. We currently have no commercial partners for this category of products and we are unlikely to develop products in this category without the financial and marketing support of a commercial partner.
Historically, we have also used plants as sources of high-quality nutritional supplements. We have a patented process for hydroponic growth of edible plants that causes them to accumulate high levels of important nutritional minerals such as chromium, selenium, iron and zinc. We will license various wholly-owned subsidiaries of Integrated BioPharma, Inc., formerly our parent company, to use our patented process for the production, marketing and sales of these phytomineral products, in exchange for a royalty based upon net sales of products utilizing our technology.
A key element of our business strategy and our thinly-staffed employment structure is to establish business arrangements with licensees, particularly leading pharmaceutical and biotechnology companies, to use our platform technology for the development and commercialization of our product candidates. As described above, FhCMB and our company are currently working within our business structure to develop product candidates based upon our plant-based platform technology. This is currently our only similar business relationship. The termination of this arrangement might adversely affect our ability to develop and commercialize our product candidates.
We rely upon FhCMB for support in advancing certain of our drug candidates and intend to rely on additional work with possible collaborators during further development and testing of our product candidates. Our possible licensees, collaborators or customers may be conducting multiple product development efforts within the same disease areas that are the subjects of their agreements with us. Agreements with customers may not preclude them from pursuing development efforts using a different approach from that which is the subject of our agreement with them. Any of our vaccine or other product candidates, therefore, may be subject to competition with a product candidate under development by a licensee or customer.
We are pursuing and obtaining non-dilutive government and non-governmental organization funding directed through FhCMB to provide supplemental capital for advancement of our programs. To date, FhCMB has been awarded a total of approximately $16 million in grants from the Bill & Melinda Gates Foundation for development of product candidates based on the iBioLaunch platform and for research and development of vaccines against influenza, malaria and African sleeping sickness (trypanosomiasis). To facilitate the grant and continuing support, we have agreed to make our platform technology available to various programs to complete development and provide so-called “Global Access” to vaccines against influenza, rabies virus, malaria and trypanosomiasis, provided that if the Gates Foundation and FhCMB do not pursue such programs to completion, the subject rights revert to us. The term “Global Access” means access for people most in need within the developing world in low income and lower-middle-income countries, as identified by the World Bank. Because we have exclusive commercial rights to these products for human health applications, this grant and any further similar grants would benefit us by enabling FhCMB to enhance the platform technology and expand the information about the technical performance of product candidates derived from the technology that we may decide to commercially license to advance into human clinical evaluation and eventual commercial development. The U.S. Department of Defense, or DoD, has also provided $14.4 million in funding to FhCMB for preclinical and clinical studies for the anthrax and plague vaccine projects, and this funding is similarly beneficial to us because of our rights to commercially exploit the technology developed.
Pursuant to the Technology Transfer Agreement between us and FhCMB, effective as of January 1, 2004, we agreed to make payments totaling $3,600,000 to FhCMB on a non-contingent basis for the acquisition of exclusive rights in intellectual property owned by FhCMB and to obtain from FhCMB maintenance and support necessary to protect the intellectual property through the preparation and filing of patent applications in the United States and around the world, of which one United States patent has been granted, one allowed, and 21 are pending. In addition 34 foreign patent applications are pending.
The intellectual property comprises the technology platform pursuant to which hydroponically grown green plants can be used for the accelerated development and manufacture of high-value proteins of interest as candidate products applicable to a broad range of disease agents, such as influenza, sleeping sickness, anthrax, plague and HPV. As of March 1, 2006, we amended this agreement to include veterinary influenza applications.
20
In addition to the acquisitions pursuant to the Technology Transfer Agreement, we have by separate agreements in the ordinary course engaged FhCMB to perform certain research activities for which we make payments when certain milestone tasks have been performed. The payments are conditioned only on the performance of the task, not upon the success or value of what is determined or discovered.
We amended our agreements with FhCMB to extend our licensing rights from 10 years to 15 years concurrent with the additional commitment to provide funding to commercialize the developed process, production techniques and methodologies of the proprietary technology and intellectual property for external applications. This amendment also requires FhCMB to conduct research to enhance, improve and expand the existing intellectual property, and for this research we have committed to make non-refundable payments of $2.0 million per year for five years, aggregating to $10.0 million, beginning November 2009. In addition, we will make royalty payments to FhCMB equal to 1% of all receipts derived by us from sales of products utilizing the proprietary technology and 15% of all receipts derived by us from licensing the propriety technology to third parties for a period of fifteen years. Minimum annual aggregate payments of $200,000 are required under the agreement beginning in 2010. In turn, FhCMB will pay us royalty payments equal to 9% of all receipts, if any, realized by FhCMB sales, licensing or commercialization of the intellectual property acquired.
We are a direct participant with FhCMB on a contract from DARPA (Defense Advanced Research Agency) of the United States Department of Defense for a $20 million project to further develop our plant-based technology platform for accelerated manufacture of vaccines and antibodies. The sub-contract is for an aggregate of $700,000 over a 27-month period which began in May 2007. Phase 1 of the sub-contract was awarded and is complete ($90,000). Phase 2 of the contract ($650,000) was awarded in February 2009. The contract will facilitate construction of a pilot manufacturing plant using our platform technology with capacity to provide sufficient materials for clinical trials.
We are also a party to a Non-Standard Navy Cooperative Research and Development Agreement, or CRADA, dated September 10, 2004, along with Naval Medical Research Center, or NMRC, and FhCMB, pursuant to which the parties agreed to collaborate in the evaluation of an anthrax vaccine for its capacity to boost pathogen-specific immune responses in individuals vaccinated against anthrax upon non-invasive oral administration. Pursuant to the CRADA, each party agrees to retain ownership of any data, copyright, trademark or patent produced by that party. However, FhCMB agreed to transfer certain patents produced pursuant to the CRADA to us, and in return we agreed to pay FhCMB up to $100,000 for its efforts upon the meeting of various milestones. Additionally, NMRC agreed to fund its own efforts associated with the CRADA. The CRADA expired by its terms on August 30, 2005, but the parties are continuing their working relationship under the agreement.
Our Product Candidates
Our short-term focus is to demonstrate the commercial value of our platform technology through its application to vaccines and therapeutics for influenza and human papilloma virus (HPV). In addition, in collaboration with FhCMB, we are also developing product candidates for the biodefense market and for infectious diseases important in the developing world. None of our product candidates have entered human clinical testing, and all of them are at a preclinical stage of development. We estimate that none of our product candidates will enter human clinical testing before the fourth calendar quarter of 2009.
Diagnostic Product for Pandemic Avian Influenza. While predicting the timing of an avian influenza pandemic is not possible, reducing the potentially devastating impact of an outbreak requires an efficient method to distinguish avian influenza infections from other respiratory diseases, including seasonal influenza. There currently are no rapid diagnostic tests available for this purpose. FhCMB has discovered an antibody that appears to distinguish highly pathogenic avian influenza strains (total of 19 strains from clades (“clade” is the technical term for category) 1, 2a and 2b) from human seasonal influenza viruses. We plan to develop this proprietary antibody with a commercial partner as a point of care diagnostic product. We do not currently have a commercial partner for this product candidate.
Seasonal Influenza Vaccine. We are developing target vaccines directed against seasonal influenza virus strains. Our vaccine candidates have shown significant promise in preclinical efficacy studies in ferrets (the preferred animal model for testing influenza products). In a recent study, we evaluated three vaccine candidate formulations in groups of eight ferrets each along with both positive and negative controls. No adverse events were seen in any animals receiving our vaccine candidates. Only one animal receiving one of our vaccine candidates showed any measurable virus shedding, which is an important measure of vaccine effectiveness. These results were as good as the results obtained with positive control animals. The immune responses and protective immunity induced by our vaccine candidates in these animal tests are equivalent to results expected from this type of test to indicate the probability of effectiveness in human subjects. More detail on these tests is available in the scientific paper published in 2008 in the journal Influenza and Other Respiratory Viruses, Volume 2, pages 33-40.
Unlike the most common method of producing vaccines against influenza, our process does not rely on chicken eggs and does not require work with whole influenza viruses. Rather, we produce subunit vaccines that are composed on only parts of the
21
protein components of the disease-causing viruses. We believe our subunit vaccines are promising for prevention of influenza infection in humans because they have been demonstrated to prevent influenza infections in ferrets. The ferret is the animal species that is typically used to evaluate a candidate influenza vaccine in laboratory tests before it is tested on humans. Our near-term objective is to complete preclinical evaluation and transition selected vaccine candidates into Phase 1 human clinical trials.
Pandemic Influenza Vaccine. We are developing vaccine candidates targeting highly pathogenic avian influenza (H5N1) viruses. These candidates have demonstrated immunogenicity and have been successfully tested in mice and ferrets for protective efficacy. Like our candidate vaccines for seasonal influenza, our candidate vaccines for avian influenza are subunit vaccines. Thus, we do not need to culture the intact avian influenza virus in order to produce our candidate vaccines. The Gates Foundation has committed significant funding to FhCMB for preclinical and clinical development of this pandemic influenza vaccine candidate using our technology. Our long term goal is to develop a combined vaccine effective for preventing both seasonal and pandemic influenza infections.
Therapeutic Antibody for Influenza. Our prototype product for treatment of patients hospitalized with avian influenza is an antibody that specifically inhibits neuraminidase activity of highly pathogenic avian influenza virus strains from clades 1 and 2. Antibodies are proteins that bind specific targets, and neuraminidase is a viral protein necessary for the spread of influenza virus. When an antibody binds neuraminidase tightly enough, it can block the function of neuraminidase and stop the spread of the virus. We have preclinical evidence that the antibody is effective against drug-resistant virus samples. This antibody has potential for prophylactic use and as a first line therapy in a flu pandemic. This antibody is in the preclinical development stage.
Therapeutic Vaccine for Human Papilloma Virus. We have commercial rights to vaccine candidates developed pursuant to our business structure based on fusing a protein component of HPV called the E7 antigen, to the LicKM protein of the bacterium Clostridium thermocellum. Several of these candidate vaccine formulations have demonstrated sufficient immune stimulation and protection from disease in mouse experiments to justify further investment in its development as a potential human therapeutic product. In experimental tests in mice, with each formulation administered to ten mice, some candidates protected all of the mice from the growth of tumors caused by the HPV virus. Additional detail on these experiments was published in 2007 in the scientific journal Vaccine, 2007 Apr 20; 25(16):3018-21.
Biodefense Products. We have commercial rights to an oral anthrax booster vaccine candidate developed by FhCMB in collaboration with the Naval Medical Research Center (NMRC). Animal tests have demonstrated safety and efficacy of this product candidate. We also have commercial rights to candidate plague vaccines that FhCMB has demonstrated to be effective in non-human primate tests in which four groups of two monkeys each were inoculated and then challenged with plague infection. Detailed results of these experiments were published in 2007 in the scientific journal Vaccine, 2007 Apr 20; 25(16):3014-7.
Under DoD sponsorship, FhCMB is also conducting rabbit and non-human primate studies on a proprietary multi-agent anthrax and plague vaccine. FhCMB also developed a proprietary antibody for potential treatment of anthrax infections. A study in non-human primates demonstrated 100% protection against challenge with anthrax spores, and dose de-escalation studies are currently underway. We have exclusive commercial rights to these product candidates for use in human health. We have not established any commercial relationships for further development of these products and are dependent on FhCMB to conduct experiments to further develop these products.
Vaccines for Developing Markets. Funding for developing-world products comes primarily from FhCMB’s collaborators, especially the Gates Foundation, and supplements the research and development payments that we make to FhCMB to advance and expand the technology to which we have exclusive commercial rights. This supplemental funding provides significant benefits in technology optimization and is synergistic with our product development programs. Through these developing world programs positive preclinical immunogenicity and efficacy results have been obtained for vaccines for HPV, trypanosomiasis and malaria.
We believe that our platform technology is well-suited for application to both vaccines and antibodies. Both vaccines and antibodies are well established in clinical practice, and the route to regulatory approval for product marketing is clear for both categories based on guidance documents issued by the FDA and available at the FDA’s website, www.fda.gov. We have focused our expertise in these product classes for two important markets, influenza and HPV. We also believe our platform is useful for the development of products for diseases of potential bioterrorism importance (most of which also are serious health problems in the developing world).
22
Influenza Market. We believe that we can achieve commercial success by applying our platform technology to the development of vaccines for prevention of influenza infections and to the development of an antibody for treatment of avian influenza. We believe that market demand for influenza vaccines and therapeutics is growing quickly, driven by the increasing pandemic threat, broader target populations who are medically recommended to be vaccinated and increased compliance by the target populations to receive vaccines. Vaccine sales in the seven major markets (US, UK, Germany, France, Italy, Spain and Japan) are expected to more than double to $4.9 billion by 2016. These estimates are based on a market analysis conducted by Datamonitor. Datamonitor also states that current manufacturing capacity is not sufficient to provide enough flu vaccine even for high-risk populations. Consequently, one of the most important challenges facing the industry is the development of novel, faster manufacturing methods that offer higher yields. We believe that, with further clinical testing and development, the iBioLaunch platform will be able to address such a critical need. We have demonstrated the efficiencies of this technology at a laboratory level by producing candidate influenza vaccines in weeks versus the months required for commercially-used chicken egg methods. The yields we have obtained in these laboratory experiments are high enough to be competitive with other methods if we can achieve the same yields and the same time efficiencies on a commercial scale. We, however, have not yet tested our technology at the scale that will be required for commercial use, nor at a scale sufficient to conclude what our commercial cost of goods will be.
Biodefense Market. In collaboration with FhCMB and future commercial partners, we expect to participate in the introduction of important new prevention and treatment products as potential countermeasures against bioterrorism threats and for use in the developing world. We do not currently have any commercial partners.
Our iBioLaunch technology is a platform that uses green plants for the accelerated development and manufacture of high value proteins of immediate interest as product candidates. We believe that our technology is applicable to a broad range of disease agents, based on laboratory experiments conducted to date. We believe we can target rapidly evolving disease agents and develop product candidates that will demonstrate high safety, potency and efficacy.
Our iBioLaunch technology consists of compositions and processes that enable growing green plants to make proteins they do not naturally make, and for these new proteins to be made fast enough and in high enough yields to facilitate the evaluation of new product candidates. We believe that we will be able to license our iBioLaunch technology to corporations that will scale it up to commercial levels to provide a means of effectively manufacturing pharmaceutical proteins and vaccines.
The iBioLaunch technology is used in a series of steps. First, normal green plants are grown for a few weeks, and at the same time, genes of interest are inserted into proprietary target DNA plasmids. A plasmid is a DNA molecule, usually circular, that can replicate inside a cell, such as a bacterial cell. These plasmids include sequences derived from plant viruses to enable easier activation of genes of interest inside living green plant tissue and also sequences derived from the bacterium, Agrobacterium tumefaciens, to enable efficient transfer of the entire vehicle into green plant tissue and activation of the genes once inside. Secondly, once both the plants and the plasmids with the new gene or genes of interest are ready, we transfer the engineered plasmids into plants by first putting them into Agrobacteria and then infusing the living Agrobacteria into growing green plants where the protein encoded by the new gene can be produced. After the transfer of bacteria into plants, the plants are grown for approximately an additional week and then the plant tissue is harvested and the desired protein or vaccine molecules are extracted and purified.
Because this entire process uses commonly available materials, we are not dependent on unique sources of raw material, nor are we limited to purchasing from single suppliers. The process is fast enough and inexpensive enough to enable more experiments to be conducted in a given period of time than can usually be conducted with slower or more expensive technology such as cultured animal cells and bioreactor methods. A more technically detailed description of this technology and its use was published in 2007 in the scientific journal Influenza and Other Respiratory Viruses, volume 1, pages 19-25. Note that in this publication, the term iBioLaunch is not used to describe the technology because that commercial designation was created after the publication of these scientific data.
Because our iBioLaunch technology has proven useful at a laboratory level in the production of high value proteins of immediate interest as product candidates, we believe it can be applied to commercial product development and biologic pharmaceutical manufacturing. Advantages of our platform technology include its short development time-frame for the harvesting of the applicable protein or vaccine molecules and applicability to a broad range of disease agents. This has enabled us, at a laboratory level, to target rapidly evolving disease agents and develop product candidates which have demonstrated high safety, potency and efficacy in laboratory animal tests.
23
The table below summarizes the results of tests conducted to date to assess the breadth of applicability of our platform technology. Some, but not all, of the listed targets are currently being pursued as product candidates by us to document the effectiveness of our platform technology.
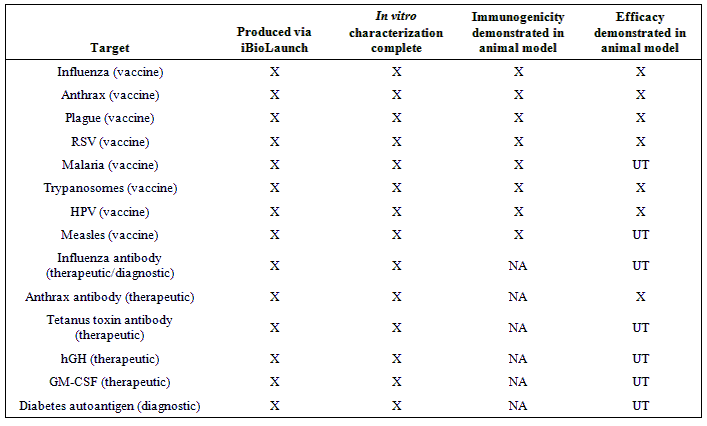
NA = not applicable UT = untested
We currently are prioritizing the following product candidates for our in-house research and development portfolio:
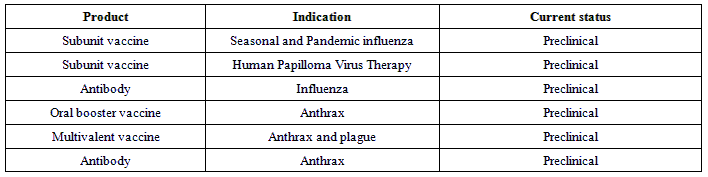
Intellectual Property
iBioPharma exclusively controls intellectual property developed at FhCMB for human health applications of plant-based production and protein expression systems. We also exclusively control the veterinary field for plant-made influenza vaccines. Our success will depend in part on our ability to obtain and maintain patent protection for our technologies and to preserve our trade secrets. Our policy is to seek to protect our proprietary rights, by among other methods, filing patent applications in the U.S. and foreign jurisdictions to cover certain aspects of our technology. For the intellectual property developed by FhCMB, we currently hold one issued U.S. patent for inducing gene silencing in plants that expires on July 25, 2022 and one U.S. patent application describing systems for expression of vaccine antigens in plants for which we have received a notice of allowance. We have an additional 19 U.S. patent applications pending. Similarly, we are preparing patent applications relating to our expanding technology for filing in the U.S. and abroad. We have also applied for patents in numerous foreign countries,
24
including Europe, Canada, Australia, China, India, Brazil, Japan, Hong Kong and New Zealand. We currently have 34 pending foreign patent applications.
The following summarizes the issued and pending patent applications on our technology and products:
Issued Technology Filing (U.S.)
|
· |
Virus-induced gene silencing in plants |
Pending Technology Filings (U.S. and International)
|
· |
Virus-induced gene silencing in plants (International) |
|
· |
Activation of transgenes in plants by viral vectors |
|
· |
Protein production in seedlings |
|
· |
Agroinfiltration of plants with launch vector |
|
· |
Transient expression of proteins in plants |
|
· |
Thermostable carrier molecule |
|
· |
Protein expression in clonal root cultures |
Pending Product Filings (U.S. and International)
|
· |
Antibodies |
|
· |
Influenza vaccines |
|
· |
Influenza therapeutic antibodies |
|
· |
Anthrax vaccines |
|
· |
Plague vaccine |
|
· |
HPV vaccines |
|
· |
Trypanosomiasis vaccine |
|
· |
Diabetes autoantigen |
|
· |
Human growth hormone |
While we have not established commercial licenses for our platform technology and while we currently have not yet entered into Phase 1 studies with any of our product candidates, we expect to commercialize our first influenza product through a business agreement with one or more larger firms. We have established no such agreements, and we currently expect to obtain Phase 2 or equivalent human clinical data before negotiating license or marketing agreements. By bearing the initial product development risk ourselves, we expect to be able to negotiate more favorable terms with our partners, and to achieve a higher return on investment, than would be possible with commercial agreements negotiated at an earlier stage of development.
FhCMB has demonstrated efficacy of an anthrax vaccine candidate and an anthrax-plague combination vaccine candidate in relevant animal model challenge studies. With funding from government sources, we plan to complete preclinical studies required for human safety evaluation. Our strategy for introduction of these products into the market includes partnership with one or more firms experienced in biodefense product commercialization and federal government procurement. We have not yet begun negotiations to obtain such a partnership arrangement.
25
We have no experience in the sales, marketing and distribution of pharmaceutical products. If in the future we fail to establish commercial licenses for our platform technology or we fail to reach or elect not to enter into an arrangement with a partner with respect to the sales and marketing of any of our future potential product candidates, we would need to develop a sales and marketing organization with supporting distribution capability in order to market such products directly.
Significant additional expenditures would be required for us to develop such an in-house sales and marketing organization.
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face competition from many different sources, including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions. Our commercial opportunities will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that we may develop based on the use of our platform technology.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, clinical trials, regulatory approvals and marketing approved products than we do. Several large pharmaceutical companies are currently already in the influenza vaccine business. Five injectable influenza vaccines are approved for use in the U.S. These include Afluria made by CSL Limited, Fluzone made by Sanofi-Pasteur, Fluarix made by GlaxoSmithKline, Flulaval made by ID Biomedical and distributed by GlaxoSmithKline, and Fluvirin made by Novartis. In addition, a nasally-administered influenza vaccine called FluMist is made by MedImmune. If we are successful in obtaining regulatory approval for our influenza vaccine candidate, we would have to compete against these large companies.
Smaller or early stage companies may also prove to be significant competitors, particularly through arrangements with large and established companies, and this may reduce the value of our platform technology for the purposes of establishing license agreements. For example, Novavax is developing vaccines for influenza, based on the use of cultured insect cells. Its candidate products are more advanced in development than ours are and have already demonstrated positive results in human clinical trials. Similarly, Medicago has announced preclinical experiments to produce influenza vaccines in green plants. Other companies, such as Vical, are attempting to develop vaccines based on the use of nucleic acids rather than proteins. If these efforts are successful in clinical trials, nucleic acid based vaccine products may compete effectively against our products and may potentially prevent us from being able to obtain commercial agreements or partnerships to enter the market.
In addition, these third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
We expect to rely upon licensees, collaborators or customers for support in advancing certain of our drug candidates and intend to rely on additional work with our collaborators during our efforts to commercialize our product candidates. Our licensees, collaborators or customers may be conducting multiple product development efforts within the same disease areas that are the subjects of their agreements with us. Agreements with collaborators may not preclude them from pursuing development efforts using a different approach from that which is the subject of our agreement with them. Any of our drug candidates, therefore, may be subject to competition with a drug candidate under development by a customer.
There are currently approved therapies for the diseases and conditions addressed by our vaccine and antibody candidates that are undergoing clinical trials and for the diseases and conditions that are subjects of our preclinical development program. For example, the drugs oseltamivir, amantadine, and zanamivir are used to treat certain influenza infections, and Merck’s vaccine to prevent HPV infection has been approved by the FDA with a similar vaccine developed by GlaxoSmithKline in late-stage development. There are also a number of companies working to develop new drugs and other therapies for diseases of commercial interest to us that are undergoing various stages of testing including clinical trials. The key competitive factors affecting the success of all of our product candidates are likely to be their efficacy, safety profile, price and convenience.
Government Regulation and Product Approval
Regulation by governmental authorities in the U.S. and other countries is a significant factor in the development, manufacture and marketing of pharmaceutical drugs and vaccines. All of the vaccine, therapeutic or diagnostic products developed from our platform technology will require regulatory approval by governmental agencies prior to commercialization. In particular, pharmaceutical drugs and vaccines are subject to rigorous preclinical testing and clinical trials and other pre-marketing approval requirements by the FDA and regulatory authorities in other countries. In the U.S., various federal, and, in some cases, state statutes and regulations, also govern or impact the manufacturing, safety, labeling, storage, record-keeping and marketing of pharmaceutical products. The lengthy process of seeking required approvals and the continuing need for
26
compliance with applicable statutes and regulations require the expenditure of substantial resources. Regulatory approval, if and when obtained for any of our product candidates, may be limited in scope, which may significantly limit the indicated uses for which our product candidates may be marketed. Further, approved drugs and manufacturers are subject to ongoing review and discovery of previously unknown problems that may result in restrictions on their manufacture, sale or use or in their withdrawal from the market. Please see “Risk Factors” for additional information on the regulatory risks we face in attempting to develop products for human use.
Before testing any compounds with potential therapeutic value in human subjects in the U.S., we must satisfy stringent government requirements for preclinical studies. Preclinical testing includes both in vitro and in vivo laboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. “In vitro” refers to tests conducted with cells in culture and “in vivo” refers to tests conducted in animals. Preclinical testing results obtained from studies in several animal species, as well as data from in vitro studies, are submitted to the FDA as part of an IND and are reviewed by the FDA prior to the commencement of human clinical trials. These preclinical data must provide an adequate basis for evaluating both the safety and the scientific rationale for the initial trials in human volunteers. In the case of candidate vaccine products, animal immunogenicity and immune protection tests must establish a sound scientific basis to believe that the product candidate may be beneficial when administered to humans.
In order to test a new biologic product or vaccine in humans in the U.S., an IND must be filed with the FDA. The IND will become effective automatically 30 days after receipt by the FDA, unless the FDA raises concern or questions about the conduct of the trials as outlined in the IND prior to that time. In this case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can proceed.
Clinical trials are typically conducted in three sequential phases, Phases 1, 2 and 3, with Phase 4 trials potentially conducted after initial marketing approval. The phases may be compressed, may overlap or may be omitted in some circumstances.
|
· |
Phase 1. After an IND becomes effective, Phase 1 human clinical trials may begin. These trials evaluate a product’s safety profile and the range of safe dosages that can be administered to healthy volunteers and/or patients, including, in some cases, the maximum tolerated dose that can be given to a trial subject with the target disease or condition. Phase 1 trials of drug candidates also determine how a drug is absorbed, distributed, metabolized and excreted by the body and the duration of its action. In the case of vaccines, human subjects are monitored for desirable immune reactions and for undesirable side effects. |
|
· |
Phase 2. Phase 2 clinical trials are typically designed to evaluate the potential effectiveness of the product in patients and to further ascertain the safety of the drug at the dosage given in a larger patient population. In the case of vaccine candidates, these tests are expected to demonstrate efficacy within the statistical limitations of the relatively small Phase 2 clinical trial study population, and further reduce concern that the product candidate may induce unwanted side effects. |
|
· |
Phase 3. In Phase 3 clinical trials, the product is usually tested in one or more controlled, randomized trials comparing the investigational new drug or vaccine to an approved form of therapy or vaccination or placebo in an expanded and well defined patient population and at multiple clinical sites. The goal of these trials is to obtain definitive statistical evidence of safety and effectiveness of the investigational new drug regimen or vaccine formulation as compared to a placebo or an approved standard therapy or vaccine in defined patient populations with a given disease and stage of illness, or exposed to a specific disease-causing agent such as a virus or bacterium. |
|
· |
Phase 4. Clinical trials are studies required of or agreed to by a sponsor that are conducted after the FDA has approved a product for marketing. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirement. These clinical trials are often referred to as Phase 3/4 post approval clinical trials. Failure to promptly conduct Phase 4 clinical trials could result in withdrawal of approval for products approved under accelerated approval regulations. |
After completion of Phase 1, 2 and 3 clinical trials, if there is substantial evidence that the drug or vaccine is safe and effective, a BLA is prepared and submitted for the FDA to review. We are not developing drugs as that term is defined by the FDA, and, therefore, if we successfully complete Phase 3 clinical trials, we would file a BLA for our vaccine or biologic candidate product. The BLA must contain all of the essential information on the product gathered to that date, including data from preclinical and clinical trials, and the content and format of a BLA must conform to all FDA regulations and guidelines. Accordingly, the preparation and submission of a BLA is a significant undertaking for a company.
27
A vaccine product for prevention of seasonal influenza must be modified frequently, usually each year, as the dominant strains of influenza virus change from season to season. Because these products must be modified so often, the regulations for their approval for marketing differ from biologic products that are not changed so frequently. FDA requirements specific to seasonal influenza vaccine products are described in the FDA document entitled “Clinical Data Needed to Support the
Licensure of Seasonal Inactivated Influenza Vaccines.” Although we plan to develop subunit vaccines for seasonal influenza rather than inactivated virus vaccines, the safety and efficacy standards of the FDA will not be less stringent than those described in the cited guidance document.
In the case of a vaccine candidate intended to be used in the event of a pandemic influenza outbreak, the requirements for regulatory approval do not include a Phase 3 clinical trial. This is because it is not ethical to subject human subjects to infection with a disease agent they would not naturally be exposed to, such as a hypothetical avian influenza strain with pandemic potential. Therefore, a vaccine candidate for this use must undergo rigorous evaluation of safety in Phase 1 and Phase 2 clinical trials, but efficacy is measured by evaluating subjects’ immune responses rather than by assessing the effectiveness of the vaccine candidate in actually preventing disease. The details of the requirements for FDA approval of a vaccine candidate such as our potential vaccine for pandemic influenza are available in the FDA publication “FDA Guidance for Industry: Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines.” A PDF copy of this publication can be downloaded from the FDA website at http://www.fda.gov/cber/gdlns/panfluvac.htm.
The FDA reviews all submitted BLAs before it accepts them for filing and may request additional information from the sponsor rather than accepting an application for filing. In this case, the application must be re-submitted with the additional information and, again, is subject to review before filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the BLA. Most applications are reviewed by the FDA within 10 months of submission. The review process is often significantly extended by the FDA through requests for additional information and clarification. The FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation but typically gives it great weight. If the FDA evaluations of both the BLA and the manufacturing facilities are favorable, the FDA may issue either an approval letter or an approvable letter, the latter of which usually contains a number of conditions that must be satisfied in order to secure final approval. If the FDA’s evaluation of the BLA submission or manufacturing facility is not favorable, the FDA may refuse to approve the application or issue a not approvable letter.
Any products we or a licensee manufactures or distributes under FDA approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the products. Drug manufacturers and their subcontractors are required to register with the FDA and, where appropriate, state agencies, and are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMPs (current Good Manufacturing Practices), which are the standards the FDA requires be met during the manufacturing of drugs and biologic products, and which impose procedural and documentation requirements upon us and any third party manufacturers we utilize.
We will also be subject to a wide variety of foreign regulations governing the development, manufacture and marketing of our product candidates. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must still be obtained prior to manufacturing or marketing the product in those countries. The approval process varies from country to country and the time needed to secure approval may be longer or shorter than that required for FDA approval. We cannot assure you that clinical trials conducted in one country will be accepted by other countries or that approval in one country will result in approval in any other country.
The product testing and clinical trial requirements that must be met before a product candidate can be marketed are substantial, time-consuming, and require investments of millions of dollars per product candidate. We must test our vaccine candidates for safety in Phase 1 clinical trials. Vaccine candidates for use in preventing disease will be administered to healthy people, and, therefore, the standards for safety and the requirement for absence of unwanted side-effects are high. In addition to demonstrating safety, we must also demonstrate that our vaccine candidates are capable of stimulating an immune response in human subjects that convinces knowledgeable scientists and physicians that the vaccine candidate is likely to be beneficial in inducing protective immunity against the disease of interest. We must then demonstrate in humans that subjects receiving our vaccine candidate develop the disease of interest at a lower rate than subjects who do not receive our candidate. In addition, when a product is already available for use in the United States, such as vaccines for prevention of influenza infection, we must demonstrate that our vaccine candidate is not inferior to the available product.
Vaccine candidates that are intended for therapeutic use, such as our candidate for treatment of HPV, must also undergo rigorous safety evaluation. Once we have satisfied FDA requirements for initial demonstration of safety, we must then prove that the vaccine candidate is capable of inducing an immune response in humans that is specific to the disease target and strong enough to be likely to provide a treatment benefit. The vaccine candidate must then be tested successfully in human volunteers with the condition to be treated, and we must demonstrate statistically significant improvements in clinical symptoms in
28
patients who receive our experimental vaccine versus those who receive standard care or a placebo in the absence of a standard treatment.
There may be uncertainty regarding regulatory requirements for developing and obtaining marketing approval for an antibody expected to treat avian influenza infections. A product such as this may be regulated similarly to an avian influenza vaccine candidate, however the animal testing requirements will probably be much more substantial and costly due to the potential safety issues associated with the higher systemic doses of antibody required to achieve a therapeutic benefit versus the lower doses of a vaccine required to achieve a protective immune response.
Our business involves exposure to potential product liability risks that are inherent in the production and manufacture of pharmaceutical products. Prior to the spin-off, we maintained product liability insurance until for sales of our phytomineral products through Integrated BioPharma’s product liability insurance policy at $5.0 million per occurrence with a $5.0 million aggregate. Our sales of phytomineral products will continue to be covered under Integrated BioPharma’s product liability policy since the manufacturing process is performed by wholly owned subsidiaries of Integrated BioPharma. We will need to purchase our own product liability insurance policy to cover any of our clinical trial and product liability risks. We anticipate that our product liability coverage will be at least comparable to our prior coverage. However,
|
· |
we may not be able to obtain product liability insurance for future trials; |
|
· |
we may not be able to obtain product liability insurance for future products; |
|
· |
we may not be able to maintain product liability insurance on acceptable terms; |
|
· |
we may not be able to secure increased coverage as the commercialization of our technology proceeds; or |
|
· |
our insurance may not provide adequate protection against potential liabilities. |
Our inability to obtain adequate insurance coverage at an acceptable cost could prevent or inhibit the commercialization of our products. Defending a lawsuit would be costly and significantly divert management’s attention from conducting our business. If third parties were to bring a successful product liability claim or series of claims against us for uninsured liabilities or in excess of insured liability limits, our business, financial condition and results of operations could be materially harmed.
As of April 15, 2009, we had six full-time employees and one part-time employee. Our employees are not represented by any union and are not the subject of a collective bargaining agreement. We believe that we have a good relationship with our employees. We expect to increase our number of employees to ten during the next 12 months. Since our business strategy is based on outsourcing our development and clinical trial work to third parties, we believe this staffing level will be sufficient to meet our needs.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Certain statements set forth under this caption constitute “forward-looking statements.” See “Forward-Looking Statements” in this prospectus for additional factors relating to such statements. The following discussion should also be read in conjunction with the Condensed Consolidated Financial Statements of the Company and Notes thereto included elsewhere herein.
Overview
iBioPharma, Inc. (formerly, InB:Biotechnologies, Inc.) (the “Company”) is a biopharmaceutical company focused on using and promoting the use of its proprietary plant-based technology platform (the “Platform”) by which targeted proteins can be produced in plants for the development and manufacture of novel vaccines and therapeutics for humans and certain veterinary applications. The Platform was invented and developed by Fraunhofer USA Center for Molecular Biotechnology (“FhCMB”), a not-for-profit translational research institution. In January 2004, we acquired from FhCMB the Platform and FhCMB’s commitment for maintenance and support necessary to further protect the intellectual property comprising the Platform, including filing and prosecuting patent applications, providing scientific support for patent counsel’s activities on behalf of the Company and otherwise to maintain in force and good standing the Company’s intellectual property rights.
Our business model contemplates that we will license the Platform to, or enter into joint ventures or other collaborative arrangements (collectively, “Licenses”) with, other parties (“Licensees”) who wish to use the Platform for the development and/or production of their own product candidates. In order to attract appropriate Licensees and increase the value of the Company’s share of such collaborative arrangements, the Company engaged FhCMB in October 2004, to perform research and development activities to apply the Platform to create a product candidate. The Company selected plant-based flu vaccine for human use as the product candidate to exemplify the value of the Platform particularly for products that require rapid, highly-scalable and economic production. Performance of this first research agreement, which required us to make payments to FhCMB against the achievement of stated research milestones, has progressed through preclinical challenge studies in the ferret model. The Company thereafter suspended further work under that research agreement in favor of having pandemic flu vaccine using its platform become the first vaccine candidate to seek FDA permission for clinical trials. The pandemic flu vaccine candidate trials are expected to be financed by a grant from the Bill and Melinda Gates Foundation to FhCMB.
In addition, in 2006, the Company engaged FhCMB to create a prototype production module for products made through the use of the platform. The purpose for this engagement was to demonstrate the ease and economy with which platform-based products could be manufactured, again in order to attract potential licensees and increase the value of the Company’s share of business arrangements. The prototype design, which encompasses the entire production process from the seeding through pre-infiltration plant growth, infiltration with agrobacteria, harvesting of plant tissue and purification of target proteins, was completed in May 2008. Fabricated equipment for the prototype is scheduled to be delivered to FhCMB by June 2009. Equipment in the facility is scheduled to be commissioned and the facility validated for current Good Manufacturing Practices (called cGMP) production in the third quarter of 2009. The facility will then be used for pilot scale production of protein targets for clinical trials of product candidates which use the Company’s platform technology.
In addition to our direct funding of FhCMB’s application of the Platform technology to our human flu vaccine product candidate, we have established arrangements (“Non-Commercial Arrangements”) among the Company, certain government entities (“GEs”), a non-governmental organization (“NGO”) and FhCMB, pursuant to which the Company grants non-commercial rights to use its Platform for the development and production by FhCMB of product candidates selected by the GEs and NGO, in consideration for grants by the GEs and NGO directly to FhCMB to fund such research and development.
Through the Company/FhCMB contracts and the Non-Commercial Arrangements (collectively, the “Business Structure”), the Company retains ownership of the intellectual property and exclusive commercial rights in the fields of human health and veterinary influenza applications of the intellectual property; but licenses or otherwise grants use rights (i) to GEs and NGO entities for not-for-profit applications of the intellectual property for the development or application of which they granted funding, and (ii) to FhCMB for research purposes and applications in other fields. This Business Structure is enabling us to obtain commercial rights to various applications of our Platform technology funded by GEs and NGOs. It also helps us demonstrate the validity and apparent value of the Platform to parties to whom we will offer licenses or collaborative opportunities. Our use of FhCMB to perform research and development work allows us to develop our product candidates, and thereby promote the value of our Platform for licensing and collaboration purposes, without bearing the full risk and expense of establishing and maintaining our own research and development staff and facilities.
Using this Business Structure, we have applied our Platform technology to create a pipeline of proprietary product candidates which we can offer to Licensees, including vaccine and therapeutic candidates against seasonal and pandemic influenza, human papilloma virus (HPV), and other pathogens of public health significance. All of our product candidates are in the preclinical
30
development stage. We sometimes refer to the Platform technology as “iBioLaunch™ technology” or the “iBioLaunch™ platform,” and we refer to the category of this technology as “plant-based technology” or as a “plant-based platform.”
In January of 2009, the Company and FhCMB agreed to suspend further preparation for clinical trials of a seasonal flu vaccine candidate and instead to focus on clinical trials of a pandemic flu vaccine candidate of interest also to the Bill & Melinda Gates Foundation, which granted FhCMB $8.7 million to fund clinical trials of the pandemic flu candidate based upon the Company’s Platform.
Historically, we have also used plants as sources of high-quality nutritional supplements. We have a patented process for hydroponic growth of edible plants that causes them to accumulate high levels of important nutritional minerals such as chromium, selenium, iron and zinc. We will license various wholly-owned subsidiaries of Integrated BioPharma, Inc., formerly our parent company, to use our patented process for the production, marketing and sales of these phytomineral products, in exchange for a royalty based upon net sales of products utilizing our technology.
Effect of Spin-off from Integrated BioPharma, Inc.
After the distribution, which occurred on August 18, 2008, the contribution of additional capital from Integrated BioPharma, our Former Parent, and the $5.0 million private placement, Integrated BioPharma owns approximately 5.4% of our common stock, and ceased to control iBioPharma.
Critical Accounting Policies and Estimates
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
|
· |
sales returns and allowances; |
|
· |
allowance for doubtful accounts; |
|
· |
valuation and recoverability of intangible assets, including the values assigned to acquired intangible assets; |
|
· |
income taxes and valuation allowances on deferred income taxes; and |
|
· |
accruals for, and the probability of, the outcome of litigation, if any. |
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Allowances for Doubtful Accounts and Sales Returns
The Company makes judgments as to its ability to collect outstanding receivables and provides allowances for the portion of receivables when collection becomes doubtful. Provisions are made based upon a specific review of all significant outstanding amounts. We continuously monitor payments from our customers and maintain allowances for doubtful accounts for estimated losses in the period they become known.
We performed a sensitivity analysis to determine the impact of fluctuations in our estimates for our allowance for doubtful accounts. As of June 30, 2008, we had an allowance for doubtful accounts of approximately $2,300, as we believe that we have minimal exposure that our customers will not pay for their outstanding receivables as of June 30, 2008. If we were in error by one percent of the account receivable balance, the impact would be $1,100 of expense. In recording any additional allowances, a respective charge against income is reflected in the general and administrative expenses and would reduce the operating results in the period in which the increase is recorded.
31
The Company’s return policy is to only accept returns for defective products. If defective products are returned, it is the Company’s agreement with its customers that the Company cure the defect and reship the product. Our policy is that when the product is shipped we make an estimate of any potential returns or allowances. As of June 30, 2008, we had estimated that a no reserve was needed as an allowance for potential returns or allowances of our sales for the fiscal year
ended June 30, 2008. If we were in error by plus or minus one percent of the sales for this period, the impact would be approximately $9,900 of additional income or expense. In recording any additional allowances, a respective charge against income is reflected in sales, net and would reduce the operating results in the period in which the increase is recorded.
Intangible Assets
The Financial Accounting Standards Board (“FASB”) has issued Statement of Financial Accounting Standards No. 142, “Goodwill and Other Intangible Assets” (“SFAS 142”). SFAS 142 requires that goodwill and intangible assets with indefinite lives no longer be amortized against earnings, but instead tested for impairment at least annually based on a fair-value approach as described in SFAS 142.
Intangible assets with finite lives are amortized over their estimated useful lives. The useful life of an intangible asset is the period over which the asset is expected to contribute directly or indirectly to future cash flows. The carrying value of intangible assets with finite lives is evaluated whenever events or circumstances indicate that the carrying value may not be recoverable. The carrying value is not recoverable when the projected undiscounted future cash flows are less than the carrying value. Tests for impairment or recoverability require significant management judgment, and future events affecting cash flows and market conditions could result in impairment losses.
If our estimated useful lives on our intangible assets are off by 10%, either the estimated useful lives should be longer or shorter than their current estimated lives, our amortization expense would be approximately $27,400 more on a per annum basis if the estimate useful lives should be shorter by 10% than our current estimates and approximately $22,100, per annum, less if the estimated useful lives should be longer by 10% of our current estimates.
Deferred Taxes
The Company accounts for income taxes pursuant to SFAS No. 109, “Accounting for Income Taxes” (SFAS 109”). SFAS 109 is an asset-and-liability approach that requires the recognition of deferred tax assets and liabilities for the expected tax consequences and events that have been recognized in the Company’s financial statements or tax returns. In the fiscal year ended June 30, 2008, the Company had net income tax expense of approximately $4,000 compared to approximately $1,000 in the fiscal year ended June 30, 2007. Our ability to recognize an income tax benefit has been dependent on the consolidated federal taxable income (loss) of Integrated BioPharma’s controlled group for federal income tax purposes. In the fiscal year ended June 30, 2008 and 2007, the controlled group of Integrated BioPharma had a taxable losses and, therefore, did not utilize any of the losses generated by us and as a stand alone taxable entity, we would have to reserve 100% of our resulting deferred tax asset generated from the net operating loss as it is more likely than not that, in the near term, we will not generate sufficient taxable income to offset our Fiscal 2008 taxable loss. Our deferred tax asset relating to our federal and state net operating losses are fully reserved in a valuation allowance account since it is more likely than not that we will not have sufficient taxable income in the near future to offset any income taxes resulting from taxable income. Since we expect that we will continue to have future losses, we do not expect to have to pay any federal income taxes and pay only any minimum taxes in the states we operate in.
General Litigation
From time to time, the Company could be a defendant or plaintiff in various legal actions which arise in the normal course of business. As such, we would be required to assess the likelihood of any adverse outcomes to these matters as well as potential ranges of probable losses. A determination of the amount of the provision required for these commitments and contingencies, if any, which would be charged to earnings, would be made after careful analysis of each matter. Any resulting provision may change in subsequent periods due to new developments or changes in circumstances. Changes in the provision could increase or decrease the Company’s earnings in the period the changes are made.
General
The Company recognizes revenue in accordance with the Securities and Exchange Commission’s Staff Accounting Bulletin 104. The Company recognizes product sales revenue, the prices of which are fixed and determinable, when title and risk of loss have transferred to the customer, when estimated provisions for product returns, charge-backs and other sales allowances are reasonably determinable, and when collectibility is reasonably assured. Accruals for these items are presented in the financial statements as reductions to sales. The Company’s net sales represent gross sales invoiced to customers, less certain related
32
charges for discounts, returns and other allowances. Cost of sales includes the cost of raw materials and overhead associated with the packaging of the products.
Recent Accounting Pronouncements
In October 2008, the FASB issued FSP No. 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active” (“FSP 157-3”). FSP 157-3 clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 was effective for us on December 31, 2008 for all financial assets and liabilities recognized or disclosed at fair value in our Condensed Financial Statements on a recurring basis (at least annually).
In March 2008, the FASB issued SFAS No. 161, “Disclosures about Derivative Instruments and Hedging Activities”, an amendment of FASB SFAS No. 133. SFAS No. 161 requires disclosure of how and why an entity uses derivative instruments, how derivative instruments and related hedged items are accounted for and how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows. SFAS No. 161 is effective for fiscal years beginning after November 15, 2008, with early adoption permitted. We do not expect SFAS No. 161 to have a material impact on our consolidated financial position, results of operations and cash flows.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements,” an amendment of ARB No. 51. The standard changes the accounting for noncontrolling (minority) interests in consolidated financial statements including the requirements to classify noncontrolling interests as a component of consolidated stockholders’ equity, and the elimination of “minority interest” accounting in results of operations with earnings attributable to noncontrolling interests reported as a part of consolidated earnings. Additionally, SFAS No. 160 revises the accounting for both increases and decreases in a parent’s controlling ownership interest. SFAS No. 160 is effective for fiscal years beginning after December 15, 2008, with early adoption prohibited. We are currently evaluating the impact of the pending adoption of SFAS No. 160 on our consolidated financial statements.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB SFAS No. 115,” which allows an entity the irrevocable option to elect fair value for the initial and subsequent measurement for certain financial assets and liabilities on an instrument-by-instrument basis. Subsequent measurements for the financial assets and liabilities an entity elects to record at fair value will be recognized in earnings. SFAS No. 159 also establishes additional disclosure requirements. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007, with early adoption permitted provided that the entity also adopts SFAS No. 157. We do not expect SFAS No. 159 to have a material impact on our consolidated financial position, results of operations and cash flows.
In September 2006, the FASB issue SFAS No. 157, “Fair Value Measurement” (“SFAS No. 157”). SFAS No. 157 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 17, 2007. In February 2008, the FASB issued FASB Staff Position No. 157-1, “Application of FASB SFAS No. 157 to FASB SFAS No. 13 and Other Accounting Pronouncements That Address Fair Value Measurements for Purposes of Lease Classification or Measurement under Statement 13 and FASB Staff Position No. SFAS 157-2, Effective Date of SFAS No. 157.” Collectively, the Staff Positions defer the effective date of SFAS 157 to fiscal years beginning after November 15, 2008, for nonfinancial assets and nonfinancial liabilities except for items that are recognized or disclosed at fair value on a recurring basis at least annually, and amend the scope of SFAS No. 157. We are currently evaluating the impact of the pending adoption of SFAS No. 157 on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), “Business Combinations.” The standard changes the accounting for business combinations including the measurement of acquirer shares issued in consideration for a business combination, the recognition of contingent consideration, the accounting for pre-acquisition gain and loss contingencies, the recognition of capitalized in-process research and development, the accounting for acquisition related restructuring liabilities, the treatment of acquisition related transaction costs and the recognition of changes in the acquirer’s income tax valuation allowance. SFAS No. 141(R) is effective for fiscal years beginning after December 15, 2008, with early adoption prohibited.
In April 2008, the FASB issued FASB Staff Position (FSP) SFAS No. 142-3, “Determination of the Useful Life of Intangible Assets”. FSP FAS No. 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142, “Goodwill and
Other Intangible Assets.” FSP SFAS No. 142-3 is effective for fiscal years beginning after December 15, 2008 and early adoption is prohibited. We are currently evaluating the impact of the pending adoption of FSP SFAS No. 142-3 on our consolidated financial statements.
33
In June 2007, the FASB ratified EITF No. 07-3, “Accounting for Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and Development Activities: (EITF No. 07-3). EITF No. 07-3 requires that nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities be deferred and capitalized and recognized as an expense as the goods are delivered or the related services are performed. EITF No. 07-3 is effective, on a prospective basis, for fiscal years beginning after December 15, 2007. The adoption of EITF No. 07-3 will not have a material impact on our consolidated financial statements.
Results of Operations
Three months ended December 31, 2008 compared to the three months ended December 31, 2007
Net Sales. Net sales for the three months ended December 31, 2008 and 2007 were $379,100 and $240,200, respectively, an increase of $138,900 or 58%. Sales under our supply agreement with Mannatech represent 51.9% of our sales in the three month period ended December 31, 2008 compared to 97.9% of our net sales in the three month period ended December 31, 2007.
For the three months ended December 31, 2008, substantially all of net sales were derived from three customers. Two of these customers, L. Perrigo Company (12.1%) (formerly, JB Laboratories, Inc.) and Natural Alternatives International (39.8%), became our customers under our supply agreement with Mannatech at the direction of Mannatech for the purpose of supplying certain raw materials in the manufacturing process of Mannatech’s nutraceutical product lines. The remaining customer, FhCMB represent 43.1% of net sales for the period ended December 31, 2008 and relates to our subcontract agreement with FhCMB under their DARPA (Defense Advanced Research Agency) grant. For the three months ended December 31, 2007, substantially all of our net sales were derived from two customers: L. Perrigo Company (39.1%) and Natural Alternatives International (58.8%) all in connection with our supply agreement with Mannatech. The loss of any of these customers would have an adverse affect on our net sales.
Cost of sales. Cost of sales increased to $195,100 for the three months ended December 31, 2008, as compared to $113,900 for the three months ended December 31, 2007. Cost of sales, as a percentage of sales, were 51.4% and 47.4%, respectively for the three months ended December 31, 2008 and 2007. The primary change in the cost of sales as a percentage of net sales is the result of an increase in our cost of sales from 50% to 90% under our manufacturing oversight contract with IHT for sales under the supply agreement with Mannatech, offset by the increase in sales from our sales under our subcontract agreement with FhCMB which has no cost of sales.
Research and Development Costs. Our research and development costs were $250,000 in the three months ended December 31, 2008 compared to none in the three months ended December 31, 2007. Research and development costs consist primarily of payments made or owed to FhCMB in reaching milestones under our research agreements with them. The increase of approximately $250,000 was primarily the result in an increase of $250,000 of payments made to FhCMB under our research agreements with them in the three months ended December 31, 2008 compared to the three months ended December 31, 2007.
Selling and Administrative Expenses. Selling and administrative expenses were $505,600 for the three months ended December 31, 2008, an increase of $25,500 as compared with $480,100 for the three months ended December 31, 2007. A tabular presentation of the changes in selling and administrative expenses is as follows:
34
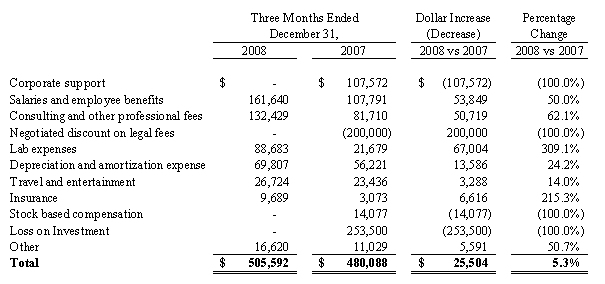
Corporate support charges from Integrated BioPharma were eliminated in the three months ended December 31, 2008 and were approximately $107,600 for the three months ended December 31, 2007. Integrated BioPharma ceased charging us the corporate support charges subsequent to the spin-off from Integrated BioPharma on August 18, 2008.
Corporate support charges consisted of the following:
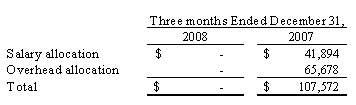
The salary allocation was an allocation of the Integrated BioPharma’s salaries and related employee costs for persons in the executive management team that devoted a portion of their time to iBioPharma’s business and an allocation of the accounting and support staff of Integrated BioPharma whom also devoted a portion of their time to our record keeping and administrative matters. The overhead allocation was an allocation of Integrated BioPharma’s allocable overhead accounts including office expenses, telephone, professional fees, consulting fees, finance charges and travel and entertainment expenses and were allocated to each of Integrated BioPharma’s subsidiaries’ based on the estimated percentage of time devoted to each company, including Integrated BioPharma, and actual expenses of Integrated BioPharma on a trailing six month period.
Salaries and employee benefits increased to $161,600 in the three months ended December 31, 2008 from $107,800 in the three months ended December 31, 2007, an increase of approximately $53,800. The increase is primarily attributable to the increased salary cost for our Chief Executive Officer and his assistant, whose salary costs were previously charged through the Corporate Support charges.
Consulting and other professional fees increased by approximately $50,700 or 62% in the three months ended December 31, 2008 to approximately $132,400 compared to approximately $81,700 in the three months ended December 31, 2007. Consulting and other professional fees consist of legal, outside accounting services, director’s fees, scientific advisory board (“SAB”) expenses (both travel and consulting fees) and consulting fees paid to outside consultants and our own Chief Scientific Officer. Additionally, in the three months ended December 31, 2007, we negotiated a discount with one of our legal firms for legal fees incurred in that period and prior periods estimated at approximately $200,000 and recorded the estimated recovery of those legal fees in the three months ended December 31, 2007 with no comparable recovery in the three months ended December 31, 2008. The increase of $50,700 in consulting and other professional fees, excluding the $200,000 recovery of legal fees, from the three months ended December 31, 2007 to December 31, 2008 was primarily the result of increases in accounting fees of $16,800, transitional services fees of $25,000 and consulting fees of $24,200, offset in part by decreases in director’s fees approximately $5,000, SAB costs of approximately $5,800 and other professional fees of $4,800. Accounting and transitional fees increased 100% in the December 31, 2008 period as these costs did not exist in the period ended December 31, 2007 as they were included in the Corporate Support Charges.
35
In the three months ended December 31, 2008, lab expense increased by $67,000 to $88,700 from $21,700 in the comparable period a year ago, $68,800 of the increase relates to salaries of employees charged to lab expense. Lab salaries increased approximately $68,800 and was the primary component of the increase in lab expenses. The increase was the result of hiring additional employees to work on lab projects, primarily related to our grant income under our FhCMB agreement, in the three
months ended December 31, 2008 compared to the year ago period.
Depreciation and amortization expense increased to approximately $69,800 in the three months ended December 31, 2008 from approximately $56,200 in the three months ended December 31, 2007, or approximately $13,600 or 24.2%. The primary increase is attributable to additional intangible assets of approximately $468,000 period over period in patents increasing our amortization expense on our patent portfolio by approximately $10,600.
Our insurance costs increased to approximately $9,700 from approximately $3,100 in the three months ended December 31, 2007, an increase of $6,600. The increase of $6,600 is primarily related to securing a separate Directors and Officers (D&O) insurance policy from our former parent. In the three months ended December 31, 2008, we had approximately $9,500 relating to this type of insurance. In the period ended December 31, 2007, our officers’ were covered under Integrated BioPharma’s D&O policy and was included in the corporate support charges in that period. This increase was offset by a decrease in product liability insurance of $2,900, as this cost is covered by IHT Health Products, Inc., a wholly owned subsidiary of our former parent, Integrated BioPharma, under our manufacturing arrangement.
Pursuant to SFAS No. 123(R), adopted as of July 1, 2005, we recognized approximately $14,100 in compensation expense for employee stock options in the three months ended December 31, 2007. This expense was a direct allocation from our Former Parent for our employees and directors who received compensation in the form of stock options providing for the purchase of our Former Parent’s stock upon vesting of their awards. There was no such expense in the three months ended December 31, 2008, as we have not issued any stock options under our plan.
In December 2006, the Company made in an investment in a private biotech company that was in its initial stages of filing to become a public company. In the three month period ended December 31, 2007, the Company, based in part on information from public filings of the biotech company, charged off its entire investment, $253,500, in this biotech company.
Other expense increased to approximately $16,600 in the three months ended December 31, 2008 from approximately $11,000 in the three months ended December 31, 2007, approximately $5,600 or 50.7%. As a percentage of total selling and administrative expenses, other expenses were 3.2% and 2.3% in the three months ended December 31, 2008 and 2007, respectively.
Income tax (benefit). In the three months ended December 31, 2008, the Company had net income tax expense of approximately $400 compared to none in the three months ended December 31, 2007. Our ability to recognize an income tax benefit was dependent on the consolidated federal taxable income (loss) of Integrated BioPharma’s controlled group for federal income tax purposes. In the three months ended December 31, 2007, the controlled group of Integrated BioPharma had a taxable loss and therefore did not utilize any of the losses generated by us and as a stand-alone taxable entity. For the three months ended December 31, 2008, we reserved 100% of our resulting deferred tax asset generated from the net operating loss as it is more likely than not that, in the near term, that we will not generate sufficient taxable income to offset our taxable losses in the periods presented. Our deferred tax asset relating to our federal and state net operating losses are fully reserved in a valuation allowance account since it is more likely than not that we will not have sufficient taxable income, in the near future, to offset any future taxable income. The income tax expense recognized in our statement of operations represents minimum state income taxes due in the states we are required to file income tax returns.
Six months ended December 31, 2008 compared to the six months ended December 31, 2007
Net Sales. Net sales for the six months ended December 31, 2008 and 2007 were $712,600 and $479,800, respectively, an increase of $232,800 or 48.5%. Sales under our supply agreement with Mannatech represent 55.1% of our sales in the six month period ended December 31, 2008 compared to 98.2% of our net sales in the six month period ended December 31, 2007.
For the six months ended December 31, 2008, substantially all of net sales were derived from three customers. Two of these customers, L. Perrigo Company (23.6%) (formerly, JB Laboratories, Inc.) and Natural Alternatives International (31.5%), became our customers under our supply agreement with Mannatech at the direction of Mannatech for the purpose of supplying certain raw materials in the manufacturing process of Mannatech’s nutraceutical product lines. The remaining customer, FhCMB represents 42.1% of net sales for the period ended December 31, 2008 and relates to our subcontract agreement with FhCMB under their DARPA (Defense Advanced Research Agency) grant. For the six months ended December 31, 2007, substantially all of our net sales were derived from two customers: L. Perrigo Company (48.8%) and Natural Alternatives
36
International (49.4%) all in connection with our supply agreement with Mannatech. The loss of any of these customers would have an adverse affect on our net sales.
Cost of sales. Cost of sales increased to $330,700 for the six months ended December 31, 2008, as compared to $231,900 for the six months ended December 31, 2007. Cost of sales, as a percentage of sales, were 46.4% and 48.3%, respectively for the six months ended December 31, 2008 and 2007. The primary change in the cost of sales as a percentage of net sales is the result of an increase in our cost of sales from 50% to 90% under our manufacturing oversight contract with IHT for sales under the supply agreement with Mannatech, offset by the increase in sales from our sales under our subcontract agreement with FhCMB which has no cost of sales.
Research and Development Costs. Our research and development costs were $500,000 in the six months ended December 31, 2008 compared to none in the six months ended December 31, 2007. Research and development costs consist primarily of payments made or owed to FhCMB in reaching milestones under our research agreements with them. The increase of approximately $500,000 was primarily the result in an increase of $500,000 of payments made to FhCMB under our research agreements with them in the six months ended December 31, 2008 compared to the six months ended December 31, 2007.
Selling and Administrative Expenses. Selling and administrative expenses were $1,002,990 for the six months ended December 31, 2008, an increase of $24,800 as compared with $978,100 for the six months ended December 31, 2007. A tabular presentation of the changes in selling and administrative expenses is as follows:
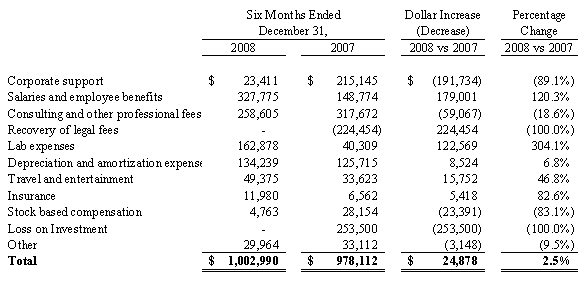
Corporate support charges from Integrated BioPharma were $23,400 in the six months ended December 31, 2008 and were approximately $215,100 for the six months ended December 31, 2007. Integrated BioPharma ceased charging us the corporate support charges subsequent to the spin-off from Integrated BioPharma on August 18, 2008.
Corporate support charges consisted of the following:
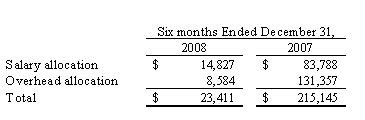
The salary allocation was an allocation of the Integrated BioPharma’s salaries and related employee costs for persons in the executive management team that devoted a portion of their time to iBioPharma’s business and an allocation of the accounting and support staff of Integrated BioPharma whom also devoted a portion of their time to our record keeping and administrative matters. The overhead allocation was an allocation of Integrated BioPharma’s allocable overhead accounts including office expenses, telephone, professional fees, consulting fees, finance charges and travel and entertainment expenses and were
37
allocated to each of Integrated BioPharma’s subsidiaries’ based on the estimated percentage of time devoted to each company, including Integrated BioPharma, and actual expenses of Integrated BioPharma on a trailing six month period.
Salaries and employee benefits increased to $327,800 in the six months ended December 31, 2008 from $148,800 in the six months ended December 31, 2007, an increase of approximately $179,000. The increase is attributable to the increased salary cost for our Chief Executive Officer and his assistant, of approximately $96,800, whose salary costs were previously charged through the Corporate Support charges, our President employed for the full six months in the period ended December 31, 2008 versus three in the year ago period resulting in an increase of $63,900 and the remaining increase of approximately was the result of salary increases and a bonus payment to other employees of approximately $118,300.
Consulting and other professional fees decreased by approximately $59,100 or 19% in the six months ended December 31, 2008 to approximately $258,600 compared to approximately $317,700 in the six months ended December 31, 2007. Consulting and other professional fees consist of legal, outside accounting services, director’s fees, scientific advisory board (“SAB”) expenses (both travel and consulting fees) and consulting fees paid to outside consultants and our own Chief Scientific Officer. Additionally, in the six months ended December 31, 2007, we negotiated discounts with our legal firms for legal fees incurred in that period and prior periods estimated at approximately $224,500 and recorded the estimated recovery of those legal fees in the six months ended December 31, 2007, with no comparable recovery in the six months ended December 31, 2008. The decrease of $59,100 in consulting and other professional fees, excluding the $224,500 recovery of legal fees, from the six months ended December 31, 2007 to December 31, 2008, was primarily the result of decreases in legal fees and other professional fees of approximately $153,200 and SAB costs of approximately $26,200, offset in part by increases accounting and audit fees of $70,600, transitional services of $36,900 and consulting fees of $12,800. Accounting and transitional fees increased 100% in the December 31, 2008 period as these costs did not exist in the period ended December 31, 2007 as they were included in the Corporate Support Charges.
In the six months ended December 31, 2008, lab expense increased by $122,600 to $162,900 from $40,300 in the comparable period a year ago, substantially all the increase relates to salaries of employees charged to lab expense. The increase in lab salaries was a result of hiring additional employees to work on lab projects, primarily related to our grant income under our FhCMB agreement, in the six months ended December 31, 2008 compared to the year ago period.
Travel and entertainment expenses increased by $15,800 to $49,400 in the six months ended December 31, 2008, from $33,600 in the six months ended December 31, 2007. This increase was the result of increased travel incurred from our president who resides in California, and our Chief Scientific Officer, who resides in London, and members of our SAB, who travel in connection with their visits to our offices in Delaware and to attend various meetings in New York and Florida in the six months ended December 31, 2008 compared to the same period in 2007.
Our insurance costs increased to approximately $12,000 from approximately $6,600 in the six months ended December 31, 2007, an increase of $5,400. The increase of $5,400 is primarily related to securing a separate Directors and Officers (D&O) insurance policy from our former parent. In the six months ended December 31, 2008, we had approximately $9,500 relating to this type of insurance. In the period ended December 31, 2007, our officers’ were covered under Integrated BioPharma’s D&O policy and was included in the corporate support charges in that period. This increase was offset by a decrease in product liability insurance of $4,000, as this cost is covered by IHT Health Products, Inc., a wholly owned subsidiary of our former parent, Integrated BioPharma, under our manufacturing arrangement.
Pursuant to SFAS No. 123(R), adopted as of July 1, 2005, we recognized approximately $4,700 in compensation expense for employee stock options in the six months ended December 31, 2008 and $28,200 in the six months ended December 31, 2007. This expense is a direct allocation from our Former Parent for our employees and directors who received compensation in the form of stock options providing for the purchase of our Former Parent’s stock upon vesting of their awards.
In December 2006, the Company made in an investment in a private biotech company that was in its initial stages of filing to become a public company. In the three month period ended December 31, 2007, the Company, based in part on information from public filings of the biotech company, charged off its entire investment, $253,500, in this biotech company.
Other expense decreased to approximately $30,000 in the six months ended December 31, 2008 from approximately $33,100 in the six months ended December 31, 2007, approximately $3,100 or 9.5%. As a percentage of total selling and administrative expenses, other expenses were 3.0% and 3.3% in the six months ended December 31, 2008 and 2007, respectively.
Income tax (benefit). In the six months ended December 31, 2008, the Company had net income tax expense of approximately $1,400 compared to $2,600 in the six months ended December 31, 2007. Our ability to recognize an income tax benefit was dependent on the consolidated federal taxable income (loss) of Integrated BioPharma’s controlled group for federal income tax purposes. In the six months ended December 31, 2007, the controlled group of Integrated BioPharma had a taxable loss and
38
therefore did not utilize any of the losses generated by us and as a stand-alone taxable entity. For the six months ended December 31, 2008, we reserved 100% of our resulting deferred tax asset generated from the net operating loss as it is more likely than not that, in the near term, that we will not generate sufficient taxable income to offset our taxable losses in the periods presented. Our deferred tax asset relating to our federal and state net operating losses are fully reserved in a valuation allowance account since it is more likely than not that we will not have sufficient taxable income, in the near future, to offset any future taxable income. The income tax expense recognized in our statement of operations represents minimum state income taxes due in the states we are required to file income tax returns.
Fiscal year ended June 30, 2008 compared to the fiscal year ended June 30, 2007
Net Sales. Net sales for the fiscal year ended June 30, 2008 and 2007 were $987,100 and $896,300, respectively, an increase of $90,800 or 10%. Sales under our supply agreement with Mannatech represent substantially all our net sales in both periods.
For the fiscal year ended June 30, 2008, approximately 92% of net sales were derived from two customers. These two customers, JB Laboratories, Inc and Natural Alternatives International, became our customers under our supply agreement with Mannatech at the direction of Mannatech for the purpose of supplying certain raw materials in the manufacturing process of Mannatech’s nutraceutical product lines. For the fiscal year ended June 30, 2007, substantially all of our net sales (98.6%) were derived from three customers: Mannatech (60.3%), Natural Alternatives International (21.4%) and JB Laboratories, Inc. (16.9%), all in connection with our supply agreement with Mannatech. The loss of any of these customers would have an adverse affect on the Company’s operations.
Cost of sales. Cost of sales increased to $485,100 for the fiscal year ended June 30, 2008, as compared to $445,700 for the fiscal year ended June 30, 2007. Cost of sales, as a percentage of sales, were 49.1% and 49.7%, respectively for the fiscal years ended June 30, 2008 and 2007.
Research and Development Costs. Our research and development costs were $550,000 in the fiscal year ended June 30, 2008 compared to $673,200 in the fiscal year ended June 30, 2007. Research and development costs consist primarily of payments made or owed to FhCMB in reaching milestones under our research agreements with them. The decrease of approximately $123,200 was primarily the result in a decrease of $100,000 of payments made to FhCMB under our research agreements with them and the lack of $23,200 payments made under a research project separate from our FhCMB relationship in the fiscal year ended June 30, 2008 compared to the fiscal year ended June 30, 2007.
Selling and Administrative Expenses. Selling and administrative expenses were $1,817,500 for the fiscal year ended June 30, 2008, an increase of $375,000 or 26% as compared with $1,442,500 for the fiscal year ended June 30, 2007. A tabular presentation of the changes in selling and administrative expenses is as follows:
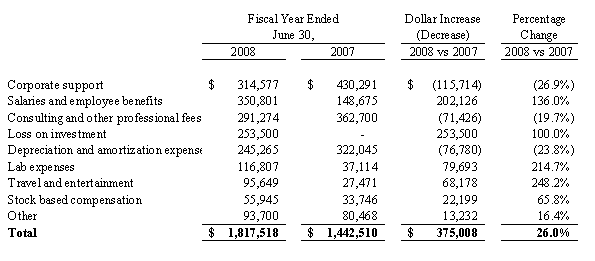
Corporate support charges from Integrated BioPharma decreased to approximately $314,600 in the fiscal year ended June 30, 2008 from approximately $430,300 from the fiscal year ended June 30, 2007, a decrease of approximately $115,700 or 27% as a result of Integrated BioPharma transferring direct payroll costs of approximately $24,000 directly to us in the fiscal year ended June 30, 2008. The remaining decrease of approximately $92,000 was the result of our Parent changing the percentage of the overhead allocation to be charged to us from 20% of allocable overhead expenses to 5% and reallocating the officers and administrative salary allocation on a lower percentage basis effective beginning January 2008. These allocations were changed mid year mostly as a result of the addition of our own president, which reduced the decrease in the allocation percentage of certain officers of Integrated BioPharma. Had the allocable percentage remained at 20%, our corporate overhead charges
39
would have been $110,400 higher. Corporate support charges ceased as of the August 18, 2008, the distribution date of the spin-off from our Parent.
Corporate support charges consisted of the following:
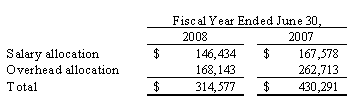
The salary allocation is an allocation of the Integrated BioPharma’s salaries and related employee costs for persons in the executive management team that devote a portion of their time to InB:Biotechnologies business and an allocation of the accounting and support staff of Integrated BioPharma whom also devote a portion of their time to our record keeping and administrative matters. The overhead allocation is an allocation of Integrated BioPharma’s allocable overhead accounts including office expenses, telephone, professional fees, consulting fees, finance charges and travel and entertainment expenses and are allocated to each of Integrated BioPharma’s subsidiaries’ based on the estimated percentage of time devoted to each company, including Integrated BioPharma, and actual expenses of Integrated BioPharma on a trailing six month period.
Salaries and employee benefits increased to $350,800 in the fiscal year ended June 30, 2008 from $148,700 in the fiscal year ended June 30, 2007, an increase of approximately $202,100. The increase is primarily attributable to the hiring of our President in October 2007 and other employees between October 2007 and June 2008, increasing our salary costs by approximately $172,300 and our employee benefit expense by approximately $29,800 in the fiscal year ended June 30, 2008 compared to no such expense in the comparable period a year ago.
Consulting and other professional fees decreased by approximately $71,400 or 19.7% in the fiscal year ended June 30, 2008 to approximately $291,300 compared to approximately $362,700 in the fiscal year ended June 30, 2007. Consulting and other professional fees consist of legal, outside accounting services, director’s fees, scientific advisory board (“SAB”) expenses (both travel and consulting fees) and consulting fees paid to outside consultants and our own Chief Scientific Officer. The decrease from the fiscal year ended June 30, 2007 to June 30, 2008 was the result of decreased legal fees of $81,000 and decreased consulting fees of $31,200, offset in part by increased SAB costs of $36,800. Our SAB costs increased by about 119%, as there was one meeting held in the fiscal year ended June 30, 2007 and two meetings were held in the fiscal year ended June 30, 2008.
In December 2006, the Company made in an investment in a private biotech company that was in its initial stages of filing to become a public company. In the three month period ended December 31, 2007, the Company, based in part on information from public filings filed in February 2008 by this biotech company, which stated that if the company was unsuccessful in its efforts to raise additional capital, it only had enough cash on hand to cover operating expenses through May 2008 and if it were successful in obtaining additional funding, such financings would have a dilutive effect to current stockholders. Furthermore, this biotech company is not a public company, the financial statements included in the public filing stated that there was substantial doubt about the company’s ability to continue as a going concern and there is no established market for the investment we hold, we therefore recorded a valuation reserve equal to our entire investment of $253,500, in this biotech company.
Depreciation and amortization expense decreased to approximately $245,300 in the fiscal year ended June 30, 2008 from approximately $322,100 in the fiscal year ended June 30, 2007, or approximately $76,800. The decrease is primarily due to an increase in the expected life of our intellectual property acquired from FhCMB from 15 years to 20 years resulting from an amendment of the FhCMB technology agreement at the end of our June 30, 2007 fiscal year end. The decrease of approximately $106,300 in our intellectual property amortization expense was offset in part, by an increase of $29,100 in our amortization expense of patents.
In the fiscal year ended June 30, 2008, lab expense increased by $79,700 to $116,800 from $37,100 in the comparable period a year ago, $41,600 of the increase relates to salaries of employees charged to lab expense. In the fiscal year ended June 30, 2008, an employee’s salary of approximately $38,000 was charged directly to lab expense as he exclusively works in the lab overseeing the production of the raw material under the Mannatech supply agreement. This employee was transferred from another wholly-owned subsidiary of Integrated BioPharma in January 2007 and was no longer charged through the corporate support allocation. In the six month period ended December 31, 2006, his salary was included in the corporate salary allocation
40
from Integrated BioPharma and in the six month periods ended June 30, 2007 and December 31, 2007, approximately $37,100 of salary expense was charged to lab expense in both periods. The increase in lab salaries of approximately $41,600 was a result of hiring additional employees who work on lab projects other than the Mannatech supply agreement in the fiscal year ended June 30, 2008. The remaining change of approximately $38,100 relates to increased supplies used by the new employees in their project work.
Travel and entertainment expenses increased by $68,200 to $95,600 in the fiscal year ended June 30, 2008, from $27,500 in the fiscal year ended June 30, 2007. This increase was the result of increased travel incurred in connection with our recruiting efforts for our newly hired president who began in October 2007, and additional travel incurred in the 2008 period in connection with our private placement efforts to raise additional capital. Additionally, our president who resides in California, and our Chief Scientific Officer, who resides in London, made several trips to our offices in Delaware and attended various meetings in New York and Florida in the fiscal year ended June 30, 2008 compared to the same period in 2007 resulting in increased travel and lodging costs of $52,600 and additional meal and entertainment costs of $15,600.
Other expense increased to approximately $93,700 in the fiscal year ended June 30, 2008 from approximately $80,500 in the fiscal year ended June 30, 2007, approximately $13,200 or 16.4%. As a percentage of total selling and administrative expenses, other expenses were 5.2% and 5.6% in the fiscal years ended June 30, 2008 and 2007, respectively, a decrease as a percentage of the total selling and administrative expenses.
Pursuant to SFAS No. 123(R), adopted as of July 1, 2005, we recognized approximately $56,000 in compensation expense for employee stock options in the fiscal year ended June 30, 2008 and $33,700 in the fiscal year ended June 30, 2007. This expense is a direct allocation from our former Parent for our employees and directors who received compensation in the form of stock options providing for the purchase of our Parent’s stock upon vesting of their awards.
Income tax (benefit). In the fiscal year ended June 30, 2008, the Company had net income tax expense of approximately $4,000 compared to $1,000 in the fiscal year ended June 30, 2007. Our ability to recognize an income tax benefit is dependent on the consolidated federal taxable income (loss) of Integrated BioPharma’s controlled group for federal income tax purposes. In the fiscal year ended June 30, 2008 and 2007, the controlled group of Integrated BioPharma had a taxable loss and therefore did not utilize any of the losses generated by us and as a stand-alone taxable entity, we would have to reserve 100% of our resulting deferred tax asset generated from the net operating loss as it is more likely than not that, in the near term, that we will not generate sufficient taxable income to offset our Fiscal 2008 and 2007 taxable losses. Our deferred tax asset relating to our federal and state net operating losses are fully reserved in a valuation allowance account since it is more likely than not that we will not have sufficient taxable income, in the near future, to offset any future taxable income.
Seasonality
We do not believe that our operations are impacted by seasonality.
Liquidity and Capital Resources
The following table sets forth, for the periods indicated, the Company’s net cash flows used in operating, investing and financing activities:
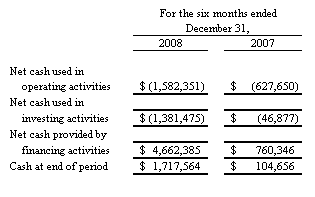
At December 31, 2008, we had working capital of $1.6 million, an increase from our negative working capital of $1.8 million as of June 30, 2008. Our cash position increased significantly from June 30, 2008 as a result of the $4.6 million of net proceeds we received from our private placement of common stock in August 2008.
41
In the six months ended December 31, 2008, we used $1.6 million of cash from our operating activities compared to $627,700 of cash in operations in the six months ended December 31, 2007, an increase of approximately $954,700. The increase of approximately $954,700 in cash used in operating activities is composed of increases in; our operating loss of $643,200 (excluding non-cash activities) (the primary increase in the operating loss was the increase in research and development
expenses of $500,000) and increases in the use of cash of $248,900 in accounts receivables, other assets of $22,900 and, a net increase in accounts payable and accrued expenses and other liabilities of $39,700.
The increase in our accounts receivable balance is a result of billing FhCMB at the end of December 2008 for work performed under our subcontract agreement relating to FhCMB’s DARPA grant in the amount of $163,400. Excluding this receivable from FhCMB, our accounts receivable balance would have increased by approximately $60,000 as a result of increased sales from the June 2008 quarter to the December 31, 2008 quarter resulting from the supply agreement with Mannatech. The net increase in accounts payable and accrued expenses of an aggregate amount of $39,700 is primarily attributable to using the proceeds from our $5.0 million private placement to pay down our outstanding liabilities that had been building over the six month period ended June 30, 2008.
The increase in cash used from investing activities of approximately $1.3 million in our six months ended December 31, 2008 from our six months ended December 31, 2007 is primarily due to the payment of $750,000 owed to FhCMB as of June 30, 2008, which we were delayed in paying until August 2008, upon the completion of our $5.0 million private placement of capital and then paying for the remaining $300,000 owed on the intellectual property relating to our iBioLaunch technology developed by FhCMB which become due in the six month period ended December 31, 2008. The balance of the increase, or $280,000, was additional spending on our patent portfolio.
The increase in cash provided from financing activities of approximately $4.2 million from six months ended December 31, 2007 to 2008, is a result of the net proceeds of $4.6 million from our completion of the $5.0 million private placement of capital completed in August 2008 offset by a net decrease in advances from our Former Parent of $678,300.
The following table sets forth the Company’s future commitments as of December 31, 2008 (Purchase Obligations represents our expected payments to FhCMB under our amended technology transfer and research agreements):
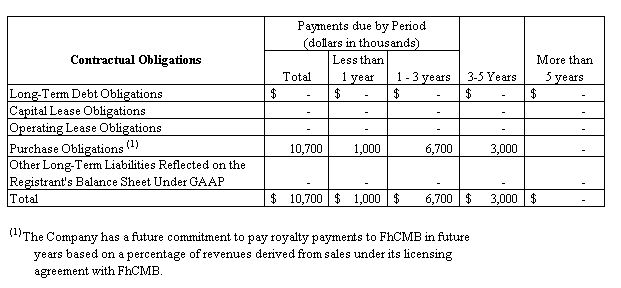
Our plans to expand our business and to continue to improve our product candidates to strengthen our ability to obtain licensees for our proprietary technology may require funds in excess of our cash flow and may require us to seek financing from third parties. In the past, Integrated BioPharma has provided capital for our general corporate purposes, and we used cash provided by Integrated BioPharma to fund our operations. Since the distribution, Integrated BioPharma has not and will not provide funds to finance our operations. Without the opportunity to obtain financing from Integrated BioPharma, we will in the future need to obtain additional financing from banks, or through public offerings or private placements of debt or equity securities, strategic relationships or other arrangements. The terms, interest rates, costs and fees of new credit facilities may not be as favorable as those historically enjoyed with Integrated BioPharma. For example, Integrated BioPharma did not charge us with any fees or costs for the intercompany borrowing, nor were there any covenants regarding financial ratios or prohibition on certain transactions in the loan arrangement with Integrated BioPharma. Our inability to obtain financing on favorable terms could restrict our operations and increase our losses.
42
In August 2008, we closed on our $5.0 million private placement, which funds were released from an escrow account subsequent to the spin-off. This additional capital is expected to cover our anticipated costs through the first quarter of calendar year 2010. If we are unsuccessful in raising additional capital or other alternative financing by then we might have to postpone or abandon our efforts to commercialize the intellectual property and suspend operations.
Capital Expenditures
The Company’s capital expenditures, other than intellectual property, during the six months ended December 31, 2008 and 2007 were not material.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
The Company does not believe that inflation has significantly affected its results of operations.
Our facilities currently consist of approximately 500 square feet of office space at our headquarters located in Newark, Delaware, which is leased on a month-to-month basis from FhCMB. In this space, we perform or maintain oversight of our administrative, clinical development, regulatory affairs and business development functions. We expect to expand our leased office space to approximately 1,500 square feet during the next 12 months, and we believe this space will be adequate to perform the same functions.
43
SECURITIES OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information with respect to the projected beneficial ownership of our outstanding common stock, immediately following the completion of the distribution, by:
|
· |
each person who is known by us to be the beneficial owner of 5% or more of our common stock; |
|
· |
each of our directors and executive officers; and |
|
· |
all of our directors and executive officers as a group. |
Except as otherwise noted in the footnotes below, the entity, individual director or executive officer or their family members or principal stockholder has sole voting and investment power with respect to such securities.
The address of each of the persons listed below is c/o iBioPharma, Inc., 9 Innovation Way, Suite 100, Newark, Delaware 19711.
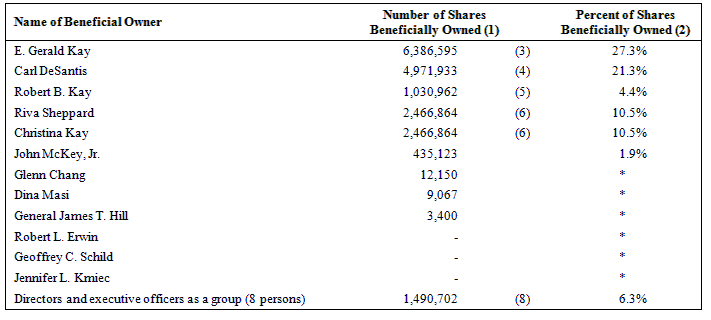
_________________________________
* Represents less than 1% of outstanding shares.
(1) Unless otherwise indicated, includes shares owned by a spouse, minor children, by relatives sharing the same home, and entities owned or controlled by the named person. Also includes shares if the named person has the right to acquire such shares within 60 days after April 15, 2009, by the exercise of warrant, stock option or other right. Unless otherwise noted, shares are owned of record and beneficially by the named person.
(2) Based upon 23,357,519 shares of common stock outstanding on April 15, 2009.
(3) Includes (i) 819,629 shares of common stock held by EGK LLC, of which Mr. Kay is the manager and (ii) 1,266,706 shares of common stock owned by Integrated BioPharma, Inc. of which Mr. Kay is a member of a control group. Shares dispositive power with Christina Kay with respect to 169,358 shares of common stock and with Riva Kay Sheppard with respect to 169,358 shares of common stock.
(4) Includes (i) 819,629 shares owned by CDS Group Holdings, LLC, of which Mr. DeSantis is the manager and (ii) 1,266,706 shares of common stock owned by Integrated BioPharma, Inc. of which Mr. DeSantis is a member of a control group.
(5) Includes 819,629 shares of common stock held by EVJ LLC, of which Mr. Kay is the manager.
(6) Includes 1,266,706 shares of common stock owned by Integrated BioPharma, Inc. of which Ms. Sheppard and Ms. Kay are members of a control group. Shares dispositive power with E. Gerald Kay with respect to 169,358 shares of common stock.
44
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Our Directors and Executive Officers
Our board of directors is currently comprised of four members; Robert B. Kay, General James T. Hill, Glenn Chang and John D. McKey, Jr., and we intend to seek additional directors with expertise in biopharmaceutical product development and marketing. Specific individuals to fill our two to four open director positions for a total of seven to nine directors have not been identified at the present time. Our board of directors is divided into three classes. Approximately one third of the directors will be Class I directors, with terms expiring at the annual meeting of stockholders to be held in 2009, approximately one third will be Class II directors with terms expiring at the annual meeting of stockholders to be held in 2010 and approximately one third will be Class III directors with terms expiring at the annual meeting of stockholders to be held in 2011. Commencing with the annual meeting of stockholders to be held in 2009, directors for each class will be elected at the annual meeting of stockholders held in the year in which the term for that class expires and thereafter will serve for a term of three years.
Our executive officers, directors and their ages as of April 15, 2009, are as follows:
There are no family relationships among any of our directors, officers or key employees.
Director and officer biographies are as follows:
Robert B. Kay is the Chief Executive Officer and a Director of our company. Mr. Kay was a founder and senior partner of the New York law firm of Kay Collyer & Boose LLP, with a particular focus on mergers and acquisitions and joint ventures. He is also a principal and Chairman of Seaway Biltmore, Inc., a hotel ownership and management company. Mr. Kay received his B.A. from Cornell University’s College of Arts & Sciences and his J.D. from New York University Law School.
Robert Erwin has served as President of iBioPharma, Inc. since October 2007. Mr. Erwin led Large Scale Biology Corporation from its founding in 1988 through 2003, including a successful initial public offering in 2000, and continued as non-executive Chairman until 2006. He served as Chairman of Icon Genetics AG from 1999 until its acquisition by a subsidiary of Bayer AG in 2006. Mr. Erwin recently served as Managing Director of Bio-Strategic Directors LLC, providing consulting services to the life sciences industry. He is currently Chairman of Novici Biotech, a private biotechnology company and a Director of Resolve Therapeutics. Mr. Erwin’s non-profit work focuses on applying scientific advances to clinical medicine, especially in the field of oncology. He is co-founder, President and Director of the Marti Nelson Cancer Foundation, Oncology. Mr. Erwin received his BS degree with Honors in Zoology and an MS degree in Genetics from Louisiana State University.
Dina L. Masi, is Interim Chief Financial Officer of our company. Ms. Masi is also the Chief Financial Officer of Integrated BioPharma, Inc. and is acting as the interim Chief Financial Officer of the company until we complete our search for this position. Ms. Masi joined Integrated BioPharma, Inc. on November 17, 2005. Previously, Ms. Masi operated a financial services consulting firm, DLM Accounting and Financial Services, LLC, providing accounting and financial services to small business owners and SEC registrants from May 2005 to November 2005. From June 2002 to December 2004, Ms. Masi served as the Chief Financial Officer and Senior Vice President of Prescott Funding, LLC, a licensed residential mortgage lender specializing in non-conforming consumer lending. Ms. Masi also served as the Chief Financial Officer and Senior Vice President of Fintek, Inc., a privately owned financial consulting services company, from July 2001 to September 2005 and as Management Information Officer from February 1998 to July 2001.
45
Geoffrey Schild, Ph.D., CBE, has served as the Chief Scientific Officer of our company since April 2005. Dr. Schild has been involved in setting global standards for quality control of vaccines and has been an active scientific contributor to the World Health Organization (WHO) and is the former Chair of WHO’s Advisory Committee on influenza composition. From 1985 to 2002, Dr. Schild was Scientific Director of the National Institute for Biological Standards and Control (NIBSC) and a member of the National Biological Standards Board in the UK. Following his retirement in 2002 until he joined us, Dr. Schild has focused on his roles as a director of the International Association for Biologicals (IABS) and Chairman of the International Society for Influenza and other Respiratory Virus Diseases (isirv).
Jennifer Kmiec has served as Vice President of Business Development and Marketing for our company since May 2006. Ms. Kmiec has over 18 years of marketing, product management and operations experience in start-up biotechnology companies. Most recently, she was Vice President of Marketing for Athena Biotechnologies. Ms. Kmiec received her MBA from the University of California, Davis. She also holds a BS degree in Biology and began her career as a virologist. Ms. Kmiec currently serves on the Board of Directors of the Delaware BioScience Association and BioStrategy Partners.
James T. Hill, U.S. Army General (ret.), has served as a director of our company since December 2005. At the time of his retirement from active duty, General Hill was the Commander of the 4-Star United States Southern Command, reporting directly to the President and Secretary of Defense. As such he led all U.S. military forces and operations in Central America, South America and the Caribbean, worked directly with U.S. Ambassadors, foreign heads of state, key Washington decision-makers, foreign senior military and civilian leaders, developing and executing United States policy. His responsibilities included management, development and execution of plans and policy within the organization including programming, communications, manpower, operations, logistics and intelligence.
Glenn Chang is a director of our company. Since 1999 he has been Director, Executive Vice President and Chief Financial Officer of the First American International Bank, Brooklyn, N.Y. Prior to the founding of the Bank he spent almost 20 years at Citibank as Vice President. Mr. Chang is a Certified Public Accountant.
John D. McKey Jr. is a director of our company. Since 2003, Mr. McKey has served as of counsel at McCarthy, Summers, Bobko, Wood, Sawyer & Perry, P.A. in Stuart, Florida, and previously was a partner from 1987 through 2003. From 1977 to 1987 Mr. McKey was a partner at Gunster Yoakley in Palm Beach, Florida. Mr. McKey received his B.B.A at the University of Georgia and his J.D. from the University of Florida College of Law.
Our scientific advisors consult with us regularly on matters relating to:
|
· |
our research and development programs; |
|
· |
the design and implementation of our clinical trials; |
|
· |
market opportunities from a clinical perspective; |
|
· |
new technologies relevant to our research and development programs; and |
|
· |
scientific and technical issues relevant to our business. |
Our principal scientific advisors are:
46

Board Committees
Our board of directors has the authority to appoint committees to perform certain management and administrative functions. Our board has constituted an audit committee comprised of Messrs. Hill and Chang.
Our board of directors has determined that Messrs. Hill, Chang and McKey are “independent directors” as such term is defined in Rule 4200(a)(15) of the NASDAQ Marketplace Rules.
Our first annual meeting of stockholders after the distribution is expected to be held in 2009. This will be an annual meeting of stockholders for the election of directors. The annual meeting will be held at our principal office or at such other place or by electronic means as permitted by the Delaware laws and on such date as may be fixed from time to time by resolution of our board of directors.
In response to recent federal legislation, we will:
|
· |
adopt a charter for the audit committee; |
|
· |
adopt a code of business conduct and ethics applicable to our directors, officers and employees; and |
|
· |
confirm that at least one member of the audit committee possesses training, education and experience in finance or accounting resulting in a level of financial sophistication as required by applicable rules. |
47
Summary Compensation Table for 2008
The table below summarizes the total compensation paid or earned by Chief Executive Officer and our Interim Chief Financial Officer and other most highly compensated executive officers who were serving as executive officers at the end of the last completed fiscal year. There were no bonuses earned or paid during fiscal 2007.
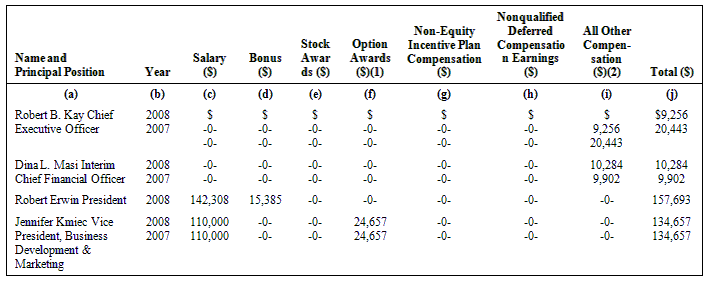
_____________________________
(1) The amounts in this column reflect the dollar amount recognized as expense with respect to stock options for financial statement reporting purposes during the twelve months ended June 30, 2008 and 2007 in accordance with SFAS No. 123(R) and thus include amounts from awards granted prior to 2007. The options are for Integrated BioPharma, Inc.’s common stock and represents the dollar amount directly allocated to iBioPharma through the Intercompany Account.
(2) The amounts in this column reflect the dollar amount charged to iBioPharma, Inc. as a component of the Corporate Support charges during the Fiscal Years ended June 30, 2008 and 2007.
Effective in August 2008, the salaries of our named executive officers are as follows:

There is currently no bonus program established.
Outstanding Equity Awards at Fiscal Year-End
There were no outstanding equity awards for the named executive officers at June 30, 2008.
48
Director Compensation
First-year director compensation for our non-employee directors will consist of a grant of 20,000 shares per annum of our common stock and cash compensation of $1,500 per quarter.
Directors who are also our employees will receive no additional compensation for their service as directors.
Director Compensation for 2008
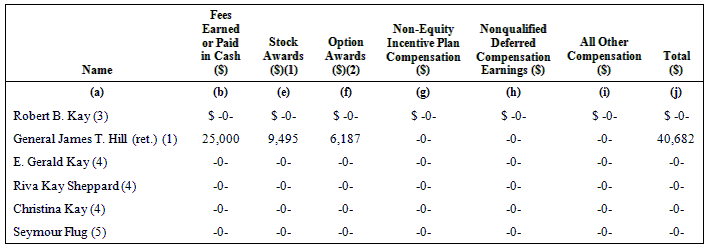
____________________________
(1) Represents the dollar amount recognized for financial statement reporting purposes with respect to fiscal year 2008 for outstanding RSUs in accordance with FAS 123R. These RSU’s were issued by our former parent, Integrated BioPharma, Inc., and were expensed in our financial statements with a corresponding amount charged to our intercompany account with Integrated BioPharma, Inc.
(2) Represents the dollar amount recognized for financial statement reporting purposes with respect to fiscal year 2008 outstanding stock options in accordance with FAS 123R. These RSU’s were issued by our Parent, Integrated BioPharma, Inc., and were expensed in our financial statements with a corresponding amount charged to our intercompany account with Integrated BioPharma, Inc.
(3) Did not receive compensation in capacity as director, but compensation as a named executive officer is disclosed above.
(4) Resigned as a member of our board of directors effective as of December 31, 2007.
(5) Resigned as a member of our board of directors effective as of September 7, 2007.
The Company currently does not have any employment contracts or other similar agreements or arrangements with any of its executive officers and does not expect to have any in place at the time of the distribution.
We expect to establish a 401(k) plan, similar to the plan in place for Integrated BioPharma, that will permit participating employees to contribute a portion of their compensation to the plan on a pre-tax basis.
We have established an incentive compensation plan and have reserved 10,000,000 shares of common stock to be issued to employees under this plan. As of December 31, 2008, no awards have been made under this plan.
49
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Historical Relationship with Integrated BioPharma, Inc.
We were a subsidiary of Integrated BioPharma from February 21, 2003 until August 18, 2008. As a result, in the ordinary course of our business, we received various services provided by Integrated BioPharma, including treasury, tax, legal, investor relations, executive oversight and other services. Integrated BioPharma also provided us with the services of a number of its executives and employees, including currently our chief financial officer. Our historical financial statements include allocations by Integrated BioPharma of a portion of its overhead costs related to these services. These cost allocations have been determined on a basis that we and Integrated BioPharma considered to be reasonable reflections of the use of these services. Integrated BioPharma allocated to us $314,600 and $430,300 in the fiscal years ended June 30, 2008 and 2007, respectively, of expenses it incurred for providing us these services.
Integrated BioPharma’s Distribution of Our Stock
As of June 30, 2008, Integrated BioPharma owned all of our common stock until completion of the distribution on August 18, 2008. In connection with the distribution, Integrated BioPharma distributed its equity interest in us to its stockholders in a transaction that was intended to be tax-free to Integrated BioPharma and its U.S. stockholders.
Agreements Between Us and Integrated BioPharma
We entered into the agreements listed below with Integrated BioPharma prior to the completion of the distribution in the context of our relationship as a subsidiary of Integrated BioPharma. The prices and other terms of these agreements may be less favorable to us than those we could have obtained in arm’s-length negotiations with unaffiliated third parties for similar services or under similar agreements.
Separation and Distribution Agreement. The separation and distribution agreement contains the key provisions relating to the distribution by Integrated BioPharma to its stockholders of our common stock.
On the distribution date, Integrated BioPharma and we entered into the following ancillary agreements governing various ongoing relationships between Integrated BioPharma and us following the distribution date:
|
· |
an indemnification and insurance matters agreement; |
|
· |
a tax responsibility allocation agreement; and |
|
· |
a transitional services agreement. |
To the extent that the terms of any of these ancillary agreements conflict with the separation and distribution agreement, the terms of these ancillary agreements govern. We describe these agreements more fully below.
Intercompany Payable. As of June 30, 2008, we were indebted to Integrated BioPharma in an amount of approximately $7.8 million, as a result of the prior intercompany financial relationship between our Company as a subsidiary and Integrated BioPharma as the corporate parent. Immediately following the consummation of the distribution, approximately $2.7 million of the then outstanding balance of the intercompany payable was converted into equity as a capital contribution to us, and, Integrated BioPharma owned 5.4% of our outstanding shares of common stock as of the August 12, 2008 when also taking into account the completion of the private placement as described herein. The remaining balance of approximately $5.0 million was contributed to capital and did not result in any new shares issued to Integrated BioPharma of iBioPharma.
Information Exchange. We and Integrated BioPharma agreed to share information with each other for use as long as no law or agreement is violated, it is not commercially detrimental to us or Integrated BioPharma, and no attorney-client privilege is waived:
|
· |
to satisfy reporting, disclosure, filing and other obligations; |
|
· |
in connection with legal proceedings other than claims that we and Integrated BioPharma have against each other; |
|
· |
to comply with obligations under the agreements between Integrated BioPharma and us; and |
50
|
· |
in connection with the ongoing businesses of Integrated BioPharma and our Company as it relates to the conduct of these businesses before the spin-off. |
Integrated BioPharma and we also agreed:
|
· |
to use reasonable commercial efforts to retain information that may be beneficial to the other; |
|
· |
and to use reasonable commercial efforts to provide the other with employees, personnel, officers or agents for use as witnesses in legal proceedings and any books, records or other documents that may be required by the other party for the legal proceedings. |
Auditing Practices. We agreed:
|
· |
to use reasonable commercial efforts to cause our auditors to date their opinion on our audited annual financial statements on the same date that Integrated BioPharma’s auditors date their opinion on Integrated BioPharma’s consolidated financial statements and to enable Integrated BioPharma to meet its timetable for the printing, filing and the dissemination to the public of any of its annual financial statements that include any financial reporting period for which our financial results are consolidated with Integrated BioPharma’s financial statements; |
|
· |
to provide Integrated BioPharma with all relevant information that Integrated BioPharma reasonably requires to enable Integrated BioPharma to prepare its quarterly and annual financial statements for quarters or years that include any financial reporting period for which our financial results are consolidated with Integrated BioPharma’s financial statements; |
|
· |
to grant Integrated BioPharma’s internal auditors access to the personnel performing our annual audits and quarterly reviews and the related work papers; and |
|
· |
not to change our accounting principles, or restate or revise our financial statements, if doing so would require Integrated BioPharma to restate or revise its financial statements for periods in which our financial results are included in Integrated BioPharma’s consolidated financial statements unless we are required to do so to comply in all material respects with generally accepted accounting principles and SEC requirements. |
Expenses. Both we and Integrated BioPharma paid our respective out-of-pocket costs and expenses incurred with respect to the distribution.
Termination and Amendment of the Agreement. Neither we nor Integrated BioPharma may terminate the separation and distribution agreement at any time after the consummation of the distribution, which was August 12, 2008, unless the other agrees.
Indemnification and Insurance Matters Agreement
Indemnification. In general, under the indemnification and insurance matters agreement, we agreed to indemnify Integrated BioPharma, its affiliates and each of its and their respective directors, officers, employees, agents and representatives from all liabilities that arise from:
|
· |
any breach by us of the separation and distribution agreement or any ancillary agreement; |
|
· |
any of our liabilities reflected on our consolidated balance sheets included in this information statement; |
|
· |
our assets or businesses; |
|
· |
the management or conduct of our assets or businesses; |
|
· |
the liabilities allocated to or assumed by us under the separation and distribution agreement, the indemnification and insurance matters agreement or any of the other ancillary agreements; |
|
· |
various on-going litigation matters in which we are named defendant, including any new claims asserted in connection with those litigations, and any other past or future actions or claims based on similar claims, facts, circumstances or events, whether involving the same parties or similar parties, subject to specific exceptions; |
51
|
· |
claims that are based on any violations or alleged violations of U.S. or foreign securities laws in connection with transactions arising after the distribution relating to our securities and the disclosure of financial and other information and data by us or the disclosure by Integrated BioPharma as part of the distribution of our financial information or our confidential information; or |
|
· |
any actions or claims based on violations or alleged violations of securities or other laws by us or our directors, officers, employees, agents or representatives, or breaches or alleged breaches of fiduciary duty by our board of directors, any committee of our board or any of its members, or any of our officers or employees. |
Integrated BioPharma agreed to indemnify us and our affiliates and our directors, officers, employees, agents and representatives from all liabilities that arise from:
|
· |
any breach by Integrated BioPharma of the separation and distribution agreement or any ancillary agreement; and |
|
· |
any liabilities allocated to or to be retained or assumed by Integrated BioPharma under the separation and distribution agreement, the indemnification and insurance matters agreement or any other ancillary agreement; |
|
· |
liabilities incurred by Integrated BioPharma in connection with the management or conduct of Integrated BioPharma’s businesses; and |
|
· |
various ongoing litigation matters to which we are not a party. |
Integrated BioPharma is not obligated to indemnify us against any liability for which we are also obligated to indemnify Integrated BioPharma. Recoveries by Integrated BioPharma under insurance policies will reduce the amount of indemnification due from us to Integrated BioPharma only if the recoveries are under insurance policies Integrated BioPharma maintains for our benefit. Recoveries by us will in all cases reduce the amount of any indemnification due from Integrated BioPharma to us.
Under the indemnification and insurance matters agreement, a party has the right to control the defense of third-party claims for which it is obligated to provide indemnification, except that Integrated BioPharma has the right to control the defense of any third-party claim or series of related third-party claims in which it is named as a party whether or not it is obligated to provide indemnification in connection with the claim and any third-party claim for which Integrated BioPharma and we may both be obligated to provide indemnification. We may not assume the control of the defense of any claim unless we acknowledge that if the claim is adversely determined, we will indemnify Integrated BioPharma in respect of all liabilities relating to that claim. The indemnification and insurance matters agreement does not apply to taxes covered by the tax responsibility allocation agreement.
Insurance Matters. Under the indemnification and insurance matters agreement, we will be responsible for obtaining and maintaining insurance programs for our risk of loss and our insurance arrangements will be separate from Integrated BioPharma’s insurance programs.
Offset. Integrated BioPharma is permitted to reduce amounts it owes us under any of our agreements with Integrated BioPharma, by amounts we may owe to Integrated BioPharma under those agreements.
Assignment. We may not assign or transfer any part of the indemnification and insurance agreement without Integrated BioPharma’s prior written consent. Nothing contained in the agreement restricts the transfer of the agreement by Integrated BioPharma.
Tax Responsibility Allocation Agreement. In order to allocate our responsibilities for taxes and certain other tax matters, we and Integrated BioPharma entered into a tax responsibility allocation agreement prior to the date of the distribution. Under the terms of the agreement, with respect to consolidated federal income taxes, and consolidated, combined and unitary state income taxes, Integrated BioPharma will be responsible for, and will indemnify and hold us harmless from, any liability for income taxes with respect to taxable periods or portions of periods ending prior to the date of distribution to the extent these amounts exceed the amounts we have paid to Integrated BioPharma prior to the distribution or in connection with the filing of relevant tax returns. Integrated BioPharma is also be responsible for, and will indemnify and hold us harmless from, any liability for income taxes of Integrated BioPharma or any member of the Integrated BioPharma group (other than us) by reason of our being severally liable for those taxes under U.S. Treasury regulations or analogous state or local provisions. Under the
52
terms of the agreement, with respect to consolidated federal income taxes, and consolidated, combined and unitary state income taxes, we are responsible for, and will indemnify and hold Integrated BioPharma harmless from, any liability for our income taxes for all taxable periods, whether before or after the distribution date. With respect to separate state income taxes, we are also responsible for, and will indemnify and hold Integrated BioPharma harmless from, any liability for income taxes with respect to taxable periods or portions of periods beginning on or after the distribution date. We are also responsible for, and will indemnify and hold Integrated BioPharma harmless from, any liability for our non-income taxes and our breach of any obligation or covenant under the terms of the tax responsibility allocation agreement, and in certain other circumstances as provided therein. In addition to the allocation of liability for our taxes, the terms of the agreement also provide for other tax matters, including tax refunds, returns and audits.
Transitional Services Agreement. The transitional services agreement we entered into with Integrated BioPharma permits us to continue to use certain corporate services previously provided to us by Integrated BioPharma as a subsidiary corporation in exchange for a management charge. After the distribution the scope of these services will be limited to legal, strategic financial planning and SEC reporting, and tax services by certain Integrated BioPharma corporate employees. In exchange for these services, we expect to pay approximately $50,000 for certain financial and tax services over an estimated period of six months.
We are not currently a party to any material legal proceedings.
53
MARKET FOR AND DIVIDENDS ON THE COMMON STOCK
Market Information
On August 18, 2008, after our most recent fiscal year, our common stock commenced trading on the OTC Bulletin Board under the symbol “IBPM.OB.” The following table shows the high and low closing prices of our common stock for the periods indicated as reported by the OTC Bulletin Board. These prices do not include retail markup, markdown or commission.

Holders
As of June 30, 2008, we were a wholly owned subsidiary of Integrated BioPharma, Inc. On the distribution date of August 18, 2008, from Integrated BioPharma, there were approximately 1,000 holders of record of our common stock.
Dividends
We have not declared or paid a dividend with respect to our common stock during the fiscal years ended June 30, 2008 or 2007 nor do we anticipate paying dividends in the foreseeable future.
Equity Compensation Plans
As of June 30, 2008, we did not currently have any shares issued under equity compensation plans.
Recent Sales of Unregistered Securities
None.
The legality of the securities offered hereby has been passed on for us by Davis Wright Tremaine LLP, New York, New York.
The financial statements for the years ended June 30, 2008 and 2007 included in this prospectus and registration statement have been so included in reliance on the report of Amper, Politziner & Mattia, LLP, an independent registered public accounting firm, as set forth in its report appearing herein and has been so included in reliance upon such report given upon the authority of such firm as experts in auditing and accounting.
Amper, Politziner & Mattia, LLP has consented to the use of its name and statements with respect to it appearing in this prospectus.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act for the common stock sold in this offering. This prospectus constitutes part of that registration statement. The registration statement contains additional information about us. We file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission. You will find additional information about us and our common stock in our Securities and Exchange Commission filings and the registration statement with respect to the statement contained in this prospectus regarding the contents of any agreement or any other document, in each instance, the statement is qualified in all respects by the text of the agreement or document, a copy of which has been filed as an exhibit to the registration statement. Our Securities and Exchange Commission filings and the registration statement and the exhibits and schedules thereto may be inspected and copied at the Securities and Exchange Commission’s Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities and Exchange Commission also maintains an Internet site
54
(http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers who file electronically with the Securities and Exchange Commission.
55
Report of Independent Registered Public Accounting Firm
We have audited the accompanying balance sheets of iBioPharma, Inc, (formerly, INB: Biotechnologies, Inc., a wholly owned subsidiary of Integrated BioPharma, Inc.) as of June 30, 2008 and 2007 and the related statements of operations, stockholder’s deficiency, and cash flows for each of the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its
internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used
and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of iBioPharma, Inc, (formerly, INB: Biotechnologies, Inc., a wholly owned subsidiary of Integrated BioPharma, Inc.) as of June 30, 2008 and 2007, and the results of its operations and its cash flows for each of the years then ended in conformity with U.S. generally accepted accounting principles.
s/ Amper, Politziner, & Mattia LLP
September 26, 2008
Edison, New Jersey
F-1
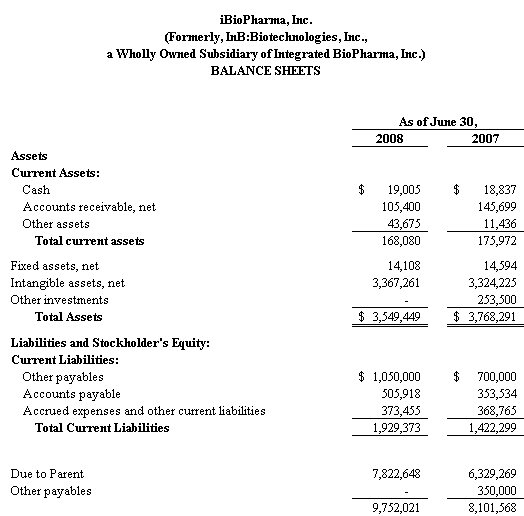
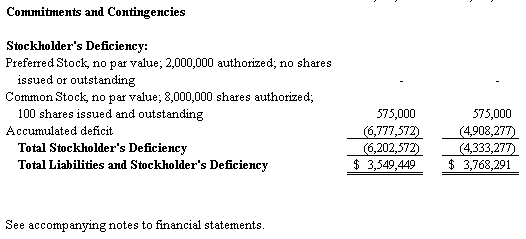
F-2
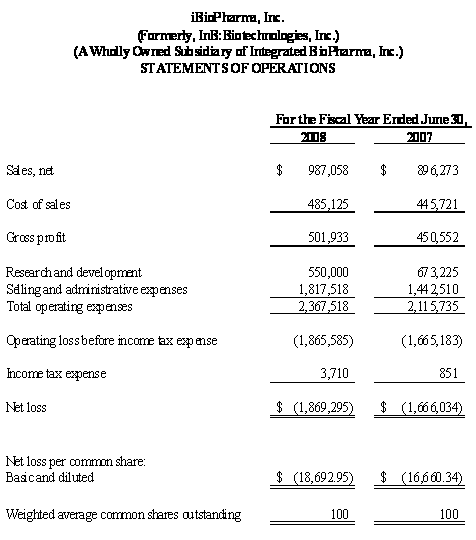
F-3
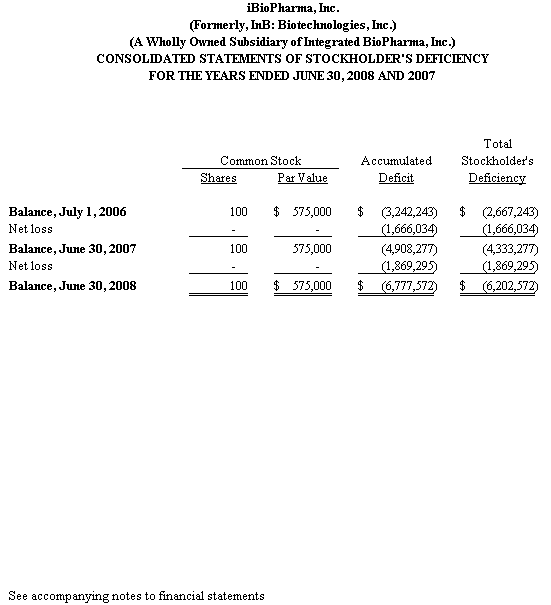
F-4

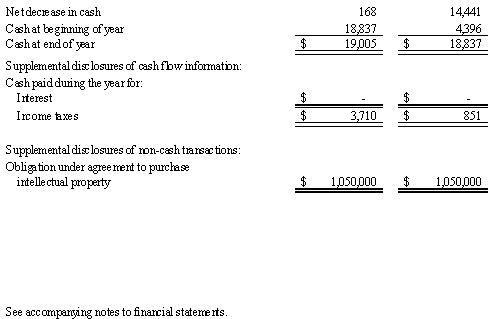
F-5
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
Note 1. Basis of Presentation and Business
iBioPharma, Inc., a Delaware Corporation, (formerly InB:Biotechnologies, Inc., a New Jersey corporation) (the “Company”) and a wholly owned subsidiary of Integrated BioPharma, Inc. (the “Parent” or “Integrated BioPharma”), is engaged primarily in the biotechnology business, which is focused on the discovery, development and commercialization of proprietary products from plants. The
Company is developing its patented plant-based expression technologies for the production of vaccines, antibodies and other therapeutic proteins. The Company is also using plants as sources of novel, high quality nutritional supplements. The Company’s patented process for the hydroponic growth of edible plants causes them to accumulate high levels of important nutritional minerals. The Company’s customers are located primarily in the United States. The Company was previously
known as Nucycle Therapy, Inc. and was incorporated on April 15, 1993 as Phytotech, Inc.
On November 9, 2007, the Board of Directors of our former Parent, approved a plan to distribute its equity interests in the Company to its stockholders. On July 25, 2008 our Parent announced the spin-off of the Company in the form of a dividend. The record date of the dividend was August 12, 2008 with a distribution date of August 18, 2008. Stockholders of our Parent received one share of the Company’s common stock for each share of common stock they owned of our Parent as of the
record date. See Note 11- Subsequent Events.
Immediately following the spin-off, the Company became a public company with stock traded on the OTC Bulletin Board under the symbol IBPM.
The Company is operating in one business segment for all periods presented.
Note 2. Summary of Significant Accounting Policies
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
|
· |
sales returns and allowances; |
|
· |
allowance for doubtful accounts; |
|
· |
valuation and recoverability of long-lived and intangible assets and goodwill, including the values assigned to acquired intangible assets; |
|
· |
income taxes and valuation allowance on deferred income taxes, and; |
|
· |
accruals for, and the probability of, the outcome of current litigation, if any. |
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Revenue Recognition. The Company recognizes revenue when the following four criteria under the Staff Accountant’s Bulletin (“SAB 104”) have been met: (i) persuasive evidence that an arrangement exists, (ii) the product has been shipped and the Company has no significant remaining obligation, (iii) the seller’s price to the buyer is fixed or determinable and (iv) collectability is reasonably assured. Among the factors the Company takes into account in determining the proper time at which to recognize revenue are when title of the goods transfers and when the risk of loss transfers. The Company’s sales policy is to require customers to provide purchase orders establishing selling prices and shipping terms. The Company evaluates the credit risk of each customer and establishes an allowance of doubtful accounts for any credit risk. Sales returns and allowances are estimated upon shipment.
Research and Development Costs. Research and development costs are expensed as incurred. The Company incurred approximately $550,000 and $673,000 in the fiscal years ended June 30, 2008 and 2007, respectively.
F-6
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
Stock-Based Compensation. As of June 30, 2008, the Company had no stock-based compensation plans. Prior to the spin-off, non-cash compensation earned by employees and directors of the Company were the result of stock options and restricted stock unit awards issued under the Parent’s stock based compensation plan.
Income Taxes. The Company had elected to file its federal income tax return as part of the consolidated federal tax return of Integrated BioPharma, its then parent company, and accordingly has not filed separate tax returns with the Internal Revenue Service since it has been a wholly owned subsidiary of Integrated BioPharma.
For state and local income taxes the Company has and continues to file tax returns separate from its Parent. The Parent and the Company account for the Company’s federal tax liabilities on the “separate company basis” method in accordance with FASB Statement No. 109, “Accounting for Income Taxes”. Under this method, the Company records tax expense and related deferred tax benefits in a manner comparable to that which it
would record if it were not affiliated with Integrated BioPharma.
The Company will file separate federal tax returns beginning in its fiscal year ending June 30, 2009, which will be for the period from August 18, 2008 to June 30, 2009, subsequent filings will be for the Company’s entire fiscal year periods ending June 30.
The Company accounts for income taxes using the liability method. Accordingly, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered
or settled. The effect on deferred tax assets and liabilities of a change in the tax rate is recognized in income or expense in the period that the change is effective. Tax benefits are recognized when it is probable that the deduction will be sustained. A valuation allowance is established when it is more likely than not that all or a portion of a deferred tax asset will either expire before the Company is able to realize the benefit, or that future deductibility is
uncertain.
Earnings Per Share. In accordance with FASB Statement No. 128, “Earnings Per Share,” basic earnings per common share are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible preferred stock, subject to antidilution limitations.
For the fiscal years ended June 30, 2008 and 2007, the Company did not have any derivative securities outstanding which would result in the dilution of earnings per share.
Fair Value of Financial Instruments. Generally accepted accounting principles require disclosing the fair value of financial instruments to the extent practicable for financial instruments which are recognized or unrecognized in the balance sheet. The fair value of the financial instruments disclosed herein is not necessarily representative of the amount that could be realized or settled, nor does the fair value amount consider the tax consequences of realization or settlement.
In assessing the fair value of financial instruments, the Company uses a variety of methods and assumptions, which are based on estimates of market conditions and risks existing at the time. For certain instruments, including cash and cash equivalents, accounts receivable, notes receivable, accounts payable, and accrued expenses, it was estimated that the carrying amount approximated fair value because of the short maturities of these instruments.
Accounts Receivable and Allowance for Doubtful Accounts. In the normal course of business, the Company extends credit to customers. Accounts receivable, less allowance for doubtful accounts, reflect the net realizable value of receivables, and approximate fair value. The Company believes there is no concentration of credit risk with any single customer whose failure or nonperformance would materially affect the Company’s results other than as discussed in Note 7(c) – Significant Risks and Uncertainties – Major Customers. On a regular basis, the Company evaluates its accounts receivables and establishes an allowance for doubtful accounts based on a combination of specific customer circumstances, credit conditions, and historical write-off and collections. The allowance for doubtful accounts as of June 30, 2008 and June 30, 2007 was $2,250. Accounts receivable are charged off against the allowance after management determines the potential for recovery is remote.
F-7
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
Fixed Assets. Fixed assets are recorded at cost and consist primarily of computer software and are amortized and depreciated over estimated useful lives of 3-5 years.
Intangible Assets. Intangible assets with finite lives are amortized over their estimated useful lives. The useful life of an intangible asset is the period over which the asset is expected to contribute directly or indirectly to future cash flows. The carrying value of intangible assets with finite lives is evaluated whenever events or circumstances indicate that the carrying value may not be recoverable. The carrying value is not recoverable when the projected undiscounted future cash flows are less than the carrying value. Tests for impairment or recoverability require significant management judgment, and future events affecting cash flows and market conditions could result in impairment losses.
Intangible assets consist of intellectual property and trademarks and patents. Amortization is being recorded on the straight-line basis over periods ranging from 10 years to 20 years based on contractual or estimated lives.
Recent Accounting Pronouncements. In March 2008, the FASB issued SFAS No. 161, “Disclosures about Derivative Instruments and Hedging Activities”, an amendment of FASB
SFAS No. 133. SFAS No. 161 requires disclosure of how and why an entity uses derivative instruments, how derivative instruments and related hedged items are accounted for and how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows. SFAS No. 161 is effective for fiscal years beginning after November 15, 2008, with early adoption permitted. We do not expect SFAS No. 161 to have a material impact on
our consolidated financial position, results of operations and cash flows.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements,” an amendment of ARB No. 51. The standard changes the accounting for noncontrolling (minority) interests in consolidated financial statements including the requirements to classify noncontrolling interests as a component of consolidated stockholders’
equity, and the elimination of “minority interest” accounting in results of operations with earnings attributable to noncontrolling interests reported as a part of consolidated earnings. Additionally, SFAS No. 160 revises the accounting for both increases and decreases in a parent’s controlling ownership interest. SFAS No. 160 is
effective for fiscal years beginning after December 15, 2008, with early adoption prohibited. We are currently evaluating the impact of the pending adoption of SFAS No. 160 on our consolidated financial statements.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB SFAS No. 115,” which allows an entity the irrevocable option to elect fair value for the initial and subsequent measurement for certain financial assets and liabilities on an instrument-by-instrument basis. Subsequent measurements for the
financial assets and liabilities an entity elects to record at fair value will be recognized in earnings. SFAS No. 159 also establishes additional disclosure requirements. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007, with early adoption permitted provided that the entity also adopts SFAS No. 157. We do not expect SFAS No. 159 to have a material impact on our consolidated financial position, results of operations and cash flows.
In September 2006, the FASB issue SFAS No. 157, “Fair Value Measurement” (“SFAS No. 157”). SFAS No. 157 defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 17, 2007. In February 2008, the FASB issued FASB Staff Position
No. 157-1, “Application of FASB SFAS No. 157 to FASB SFAS No. 13 and Other Accounting Pronouncements That Address Fair Value Measurements for Purposes of Lease Classification or Measurement under Statement 13 and FASB Staff Position No. SFAS 157-2, Effective Date of SFAS No. 157.” Collectively, the Staff Positions defer the effective date of SFAS 157 to fiscal years
beginning after November 15, 2008, for nonfinancial assets and nonfinancial liabilities except for items that are recognized or disclosed at fair value on a recurring basis at least annually, and amend the scope of SFAS No. 157. We are currently evaluating the impact of the pending adoption of SFAS No. 157 on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), “Business Combinations.” The standard changes the accounting for business combinations including the measurement of acquirer shares issued in consideration for a business combination, the recognition of contingent consideration, the accounting for pre-acquisition gain and loss contingencies, the recognition of capitalized in-process research and development, the accounting for acquisition related restructuring liabilities, the treatment of acquisition related transaction costs and the recognition of changes in the acquirer’s income tax valuation allowance. SFAS No. 141(R) is effective for fiscal years beginning after December 15, 2008, with early adoption prohibited.
F-8
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
In April 2008, the FASB issued FASB Staff Position (FSP) SFAS No. 142-3, “Determination of the Useful Life of Intangible Assets”. FSP FAS No. 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets.” FSP SFAS No. 142-3 is effective for fiscal years beginning after December 15, 2008 and early adoption is prohibited. We are currently evaluating the impact of the pending adoption of FSP SFAS No. 142-3 on our consolidated financial statements.
In June 2007, the FASB ratified EITF No. 07-3, “Accounting for Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and Development Activities: (EITF No. 07-3). EITF No. 07-3 requires that nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities be deferred and capitalized and recognized as an expense as the goods are delivered or the related services are performed. EITF No. 07-3 is effective, on a prospective basis, for fiscal years beginning after December 15, 2007. The adoption of EITF No. 07-3 will not have a material impact on our consolidated financial statements.
Note 3. Intangible Assets and Other Payables
The carrying amount of intangible assets as of June 30, 2008 and 2007 is as follows:

Intellectual property consists of exclusive licensing rights, patents and other technology relating to producing human health and veterinary influenza applications of the plant-based technology developed by the Center for Molecular Biotechnology of Fraunhofer USA, Inc. (“FhCMB”).
Under a Technology Transfer Agreement (the “TTA”) effective as of January 1, 2004, we acquired from FhCMB: (i) exclusive commercial rights to certain intellectual property invented and developed by FhCMB by which targeted proteins can be produced in plants for the development and manufacture of novel vaccines and therapeutics for humans and certain veterinary applications, and (ii) FhCMB’s commitment for maintenance and support services necessary to further protect the Platform, including filing and prosecuting patent applications, providing scientific support for patent counsel’s activities on behalf of the Company and otherwise to maintain in force and good standing the Company’s intellectual property rights. The total contract price for the Platform and the support and maintenance services was $3.0 million. In March 2006, and December 2007, the Company expanded the rights acquired from Fraunhofer to include veterinary and diagnostic applications of the Platform, for $500,000 and $100,000, respectively, which increased the original purchase price from $3.0 million to $3.6 million.
The Company recorded the payments under the TTA and payments to patent counsel for protection of the Platform as intangible assets with a definite life using the payments made to determine the fair value of the intellectual properties acquired. The Company recorded the payments at the due dates provided in the TTA after knowing that Fraunhofer had provided the required maintenance and support services in that period. When the parties entered into the TTA, we expected the articulation and filing of U.S. patent and other intellectual property protections to be accomplished substantially evenly over the term of the TTA. However, by June 30, 2007, when the Company determined that substantially all of the maintenance and support activities had been performed in support of the Platform because all of the patents and foreign applications contemplated to be filed to protect the Platform had been completed, the Company booked the remainder of the payments due under the TTA.
During the fiscal years ended June 30, 2008 and 2007, the Company made payments of $100,000 and $600,000, respectively, under an intellectual property acquisition agreement, as amended, with FhCMB entered into in January 2004. As of June 30, 2008 and 2007, the Company has a remaining commitment of $1,050,000 that will be paid in the fiscal year ending June 30, 2009 and is included in other payables at June 30, 2008 and 2007. Amortization expense
F-9
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
recorded on intangible assets for the fiscal years ended June 30, 2008, 2007 and 2006 was approximately $245,000 and $289,000, respectively. Amortization expense is recorded on the straight-line method over periods ranging from 10 years to 20 years and is included in selling and administrative expenses.
The estimated annual amortization expense for intangible assets for the five succeeding fiscal years is as follows as of June 30, 2008:
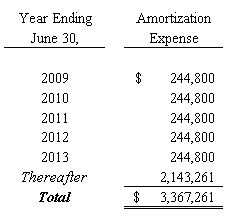
Note 4. Due to Parent
Due to Parent consists of net cash advances from Parent to assist the Company in meeting its obligations and for corporate support charges, offset by the Parent’s use of the Company’s federal net operating loss, see Note 5. The Parent did not charge the
Company interest on any of these advances. These advances consisted of the following:

The corporate overhead allocation due our Parent are allocated based on the estimated time that the Parent’s officers and employees dedicate to our Company’s business and includes charges for employee salaries and benefits, legal, accounting and other consulting fees, treasury and tax services and general office expenses. The allocations are based on actual costs incurred by our Parent.
Note 5. Income Taxes
Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial accounting purposes and the amounts used for income tax reporting. Significant components of the Company’s deferred tax assets as of June 30, 2008 and 2007 follow:
F-10
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
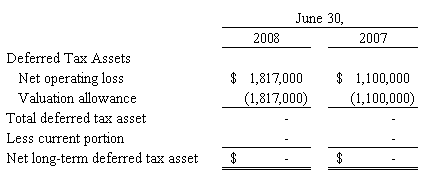
Federal net operating losses of approximately $1.5 million were used by Integrated BioPharma and are not available to the Company. The Company recognized a federal income tax benefit of $485,811 in the fiscal year ended June 30, 2006 and $17,600 in prior years for the use of the federal net operating losses by the consolidated group and reduced the amount due to its Parent accordingly. Its
Parent allocates the use of the federal net operating losses available for use on its consolidated Federal tax return on a pro rata basis based on all of the available net operating losses from all the entities included in the control group.
Federal and state net operating losses of approximately $4.3 million and $5.8 million are available to the Company and will expire beginning in 2008 through 2028. These carryforwards could be subject to certain limitations in the event there is a change in control of the Company and have been fully reserved in the Company’s valuation allowance account as there is substantial doubt the Company would be able use these net operating losses to offset future taxable income before the
net operating losses expire and the Company is able to realize the related benefit.
The components of the provision for income taxes consists of the following:
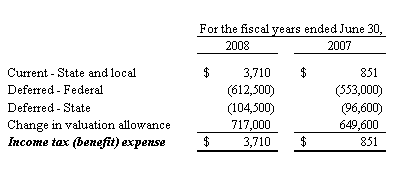
A reconciliation of the statutory tax rate to the effective tax rate is as follows:
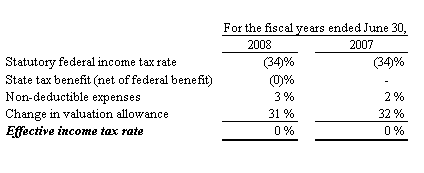
Note 6. Profit-Sharing Plan
The Company is currently included in Integrated BioPharma’s profit-sharing plan, which qualifies under Section 401(k) of the Internal Revenue Code, covering all nonunion employees meeting age and service requirements. Contributions are determined by matching a percentage of employee contributions. The total expense for the fiscal years ended June 30, 2008 and 2007 was approximately $5,000 and $6,000, respectively.
F-11
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
Note 7. Significant Risks and Uncertainties
(a) Concentrations of Credit Risk-Cash. The Company maintains balances at a financial institution. Deposit accounts at the institution are insured by the Federal Deposit Insurance Corporation for deposits up to $100,000. As of June 30, 2008, the Company had no uninsured cash balances.
(b) Concentrations of Credit Risk-Receivables. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk of its customers, establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowances is limited. The Company does not require collateral in relation to its trade accounts receivable credit risk. The amount of the allowance for uncollectible accounts and other allowances as of June 30, 2008 and 2007 was $2,250. The Company’s bad debt expense for the fiscal years ended June 30, 2008 and 2007 were none and $2,250, respectively.
(c) Major Customers. For the fiscal year ended June 30, 2008 approximately 50.6% and 41.5% of revenues were derived from two customers. For the fiscal year ended June 30, 2007 approximately 44.7%, 27.4% and 26.8% of revenues were derived from three customers. The loss of any of these customers would have an adverse affect on the Company’s operations. Accounts receivable from these customers represented 53% of the accounts receivable balance as of June 30, 2008.
(d) Major Supplier and Related Party. The Company has subcontracted the manufacturing, including the oversight of its supply agreement with a wholly owned subsidiary of Integrated BioPharma (IHT Health Products, Inc. (“IHT”)), who in turns contracts with another wholly owned subsidiary of Integrated BioPharma, substantially all of our cost of goods sold are paid to this related party. For the fiscal years ended June 30, 2008 and 2007, the Company was invoiced by IHT $484,500 and $422,800, respectively under this arrangement and such amounts are included in cost of goods sold in the accompanying statements of operations. The Company is not direct billed by the other related party utilized under the manufacturing arrangement.
(e) Other Business Risks. The Company insures it business and assets against insurable risks, to the extent that it deems appropriate, based upon an analysis of the relative risks and costs. The Company believes that the risk of loss from non-insurable events would not have a material adverse effect on the Company’s operations as a whole.
Note 8. Commitments and Contingencies
(a) Leases. The Company leases office space on a month-to-month basis. The lease was effective October 1, 2006 and provides for a minimum monthly rental of $1,126. Total rent expense, including real estate taxes and maintenance charges, was approximately $13,500 for each of the years ended June 30, 2008 and 2007.
(b) Intellectual Property and Research Agreements. In connection with the acquisition in January 2004 of intellectual property developed by the Center for Molecular Biotechnology of Fraunhofer USA, Inc. (“FhCMB”), the Company entered into a Technology Transfer Agreement on December 18, 2003 (the “IP Agreement”), whereby the Company agreed to pay up to a maximum of $3.0 million for
certain technology developed by FhCMB over a five-year period. In addition to the IP Agreement, the Company entered into research agreements, which require the payment of several milestone payments related to achieving certain flu vaccine studies and our ongoing Anthrax studies (the “R&D Agreements”).
In March, 2006, the Company amended their IP Agreement with FhCMB to expand the scope of the IP Agreement and increased the amount of the purchase commitment to a maximum of $3.5 million. In June 2007, the Company amended their existing amended IP Agreement and R&D Agreements with FhCMB, to commercialize the developed process, production techniques and methodologies of the proprietary technology and intellectual property for external applications. The June 2007 amendment requires
FhCMB to continue to conduct research to enhance, improve and expand the existing intellectual property, and for this research the Company has committed to make non-refundable payments of $2.0 million per year for five years, aggregating to $10.0 million, beginning in November 2009. In addition, the Company will make royalty payments to FhCMB based on receipts derived by the Company from sales of products utilizing the proprietary technology for a period of fifteen years instead of the
original the ten-year period. In turn, FhCMB shall pay the Company royalty payments for all receipts, if any, realized by FhCMB sales, licensing or
F-12
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
commercialization of the intellectual property acquired by them for the same fifteen-year period. Furthermore, FhCMB has agreed to expend at a minimum, an additional $2.0 million per year in the same timeframe as the Company for research and development on the intellectual property. A managing director of FhCMB is also a director on our Board and our Parent’s Board of Directors.
In December 2007, the Company and FhCMB further amended the IP Agreement increasing the purchase price by $100,000 to amend the field to include influenza diagnostics for a maximum purchase price of $3.6 million.
As of June 30, 2008 and 2007, the Company has made payments of approximately $2.6 million and $2.5 million, respectively for the purchase commitment of $3.6 million, of which $1.05 million is accrued and is to be paid in fiscal year 2009.
Under the Company’s R&D Agreements, if FhCMB achieves each of the targeted Milestones, as defined in the agreements, the Company will incur research and development costs of $1.2 million in addition to the $10.0 million under the amended IP Agreement over the course of the next five years.
Note 9. Equity Transactions
In connection with the Company entering into a Separation and Distribution Agreement (the “Distribution”) with its Parent in November 2007, the Company will restate its stockholder’s deficiency to reflect the Distribution transaction, whereby, the Parent has agreed to distribute, pro rata, to the holders of its common stock, all of the shares of the
Company’s common stock owned by Integrated BioPharma.
The completion of the Distribution was subject to various customary closing conditions, including the declaration by the U.S. Securities and Exchange Commission of the effectiveness of the registration under the Securities Exchange Act of 1934 of the Company’s common stock. The Distribution was completed on August 18, 2008. The Distribution should qualify as a tax-free reorganization under Section 355 of the Internal Revenue Code of 1986, as amended. The Agreement prohibits the
Company from issuing any additional shares of its common stock in excess of the shares issued with respect to the Distribution for the two years immediately following the effective date of the Distribution. See Note 10. Subsequent Events.
Note 10. Subsequent Events
As disclosed in Note 9, in November 2007, the Company entered into a Separation and Distribution Agreement (the “Distribution”) with its Parent, whereby, the Parent agreed to distribute, pro rata, to the holders of its common stock, all of the shares of the Company’s common stock owned by Integrated BioPharma. The completion of the Distribution was subject to various customary closing conditions,
including the declaration by the U.S. Securities and Exchange Commission of the effectiveness of the registration under the Securities Exchange Act of 1934 of the Company’s common stock. The Distribution was completed on August 18, 2008 and each shareholder of our Parent received one share of the Company for each share the shareholder owned as of August 12, 2008, the Record Date. The Distribution should qualify as a tax-free reorganization under Section 355 of the Internal Revenue
Code of 1986, as amended. The Agreement prohibits the Company from issuing additional shares of its common stock in excess of the shares issued with respect to the Distribution for the two years immediately following the effective date of the Distribution.
In August 2008, the Company entered into a Transitional Services Agreement (the “TS Agreement”) with Integrated BioPharma. The transitional services agreement permits us to continue to use certain corporate services previously provided to us by Integrated BioPharma as a subsidiary corporation in exchange for a management charge. The scope of these services is limited to legal, strategic financial planning and SEC reporting, and tax services by certain Integrated BioPharma corporate employees. In exchange for these services, the Company expect to pay approximately $50,000 for certain financial and tax services over an estimated period of six months; the TS Agreement provides for a per annum fee of $100,000.
Also as disclosed in Note 9, on August 19, 2008, our Parent entered into a Conversion Agreement, whereby the Parent caused approximately $5.2 million of the intercompany debt to be contributed to additional paid in capital and used $2.7 million of the intercompany debt to purchase approximately 1.3 million shares of the Company, representing 6% of the then outstanding shares of the Company. Subsequent to the Company’s private placement as discussed below, Integrated BioPharma owns 5.4% of the Company.
F-13
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
Additionally, on August 19, 2008, the Company closed on its $5.0 million capital raise in connection with its private placement of approximately ten percent (10%) of the Company, such funds were released to the Company from the escrow and issued approximately 2.3
million shares of the Company’s par value $0.001 common stock, at an estimated purchase price of approximately $2.13 per share.
The Company also issued to the private placement investors, warrants to purchase a number of shares of common stock equal to 50% of the number of shares purchased by such private placement investor, with an exercise price equal to 150% of the purchase price of the Company’s common stock subject to adjustments therein and warrants to purchase a number of shares of common stock equal to 50% of the number of shares purchased by such private placement investor, with an exercise price
equal to 200% of the purchase price of the Company’s common stock subject to adjustments therein and exercisable over the next five-year period.
The following table sets for the Company’s capitalization on an actual basis as of June 30, 2008, and as adjusted to give effect to the above transactions as though they had been completed on June 30, 2008:
F-14
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
(A Wholly Owned Subsidiary of Integrated BioPharma, Inc.)
NOTES TO FINANCIAL STATEMENTS
AS OF JUNE 30, 2008 AND 2007 FOR THE
FISCAL YEARS ENDED JUNE 30, 2008 and 2007
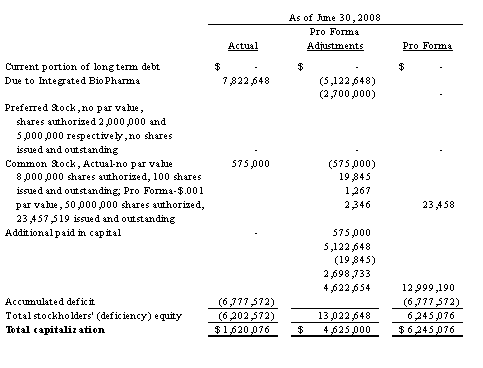
F- 15
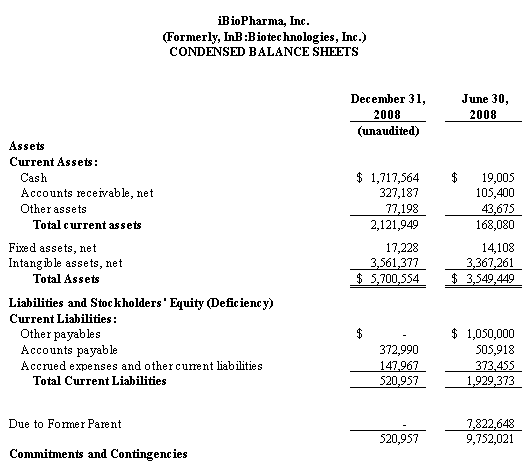
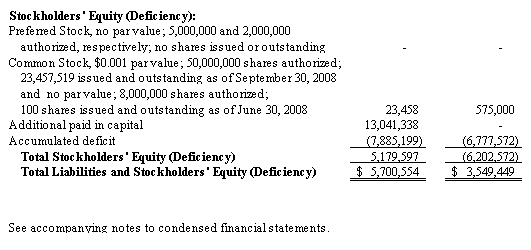
F-16
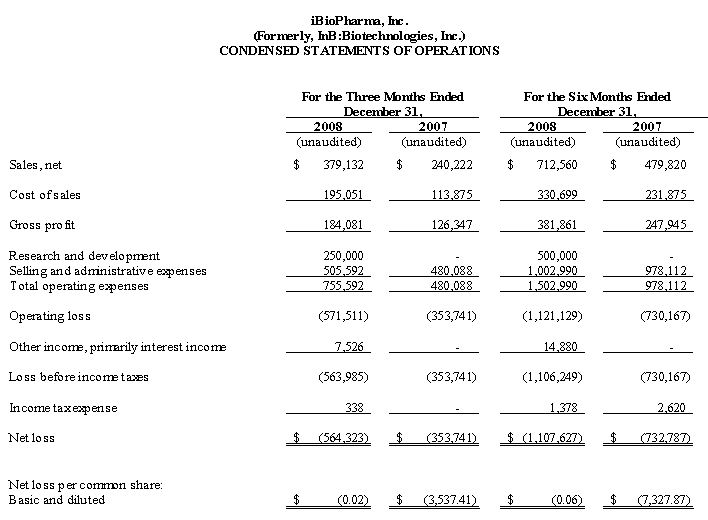

F-17
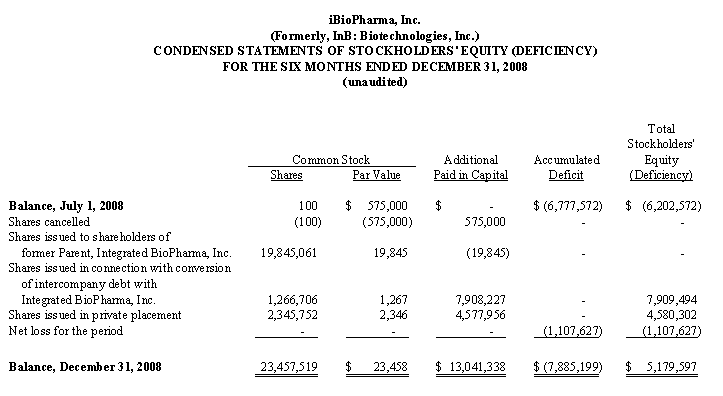
F-18
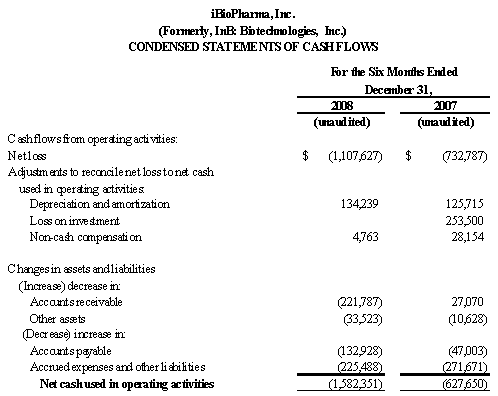
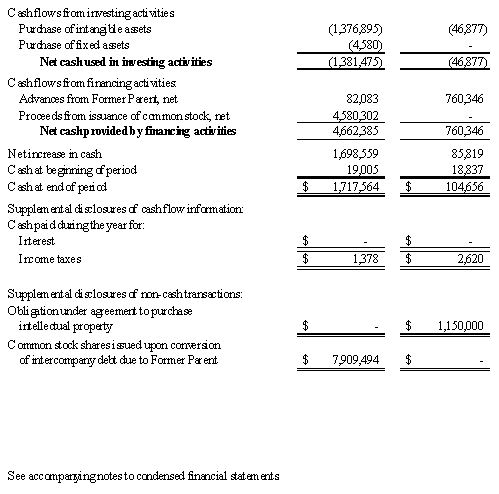
F-19
Note 1. Principles of Consolidation and Basis of Presentation and Liquidity
The accompanying financial statements for the interim periods are unaudited and include the accounts of the Company. The interim financial statements have been prepared in conformity with Rule 10-01 of Regulation S-X of the Securities and Exchange Commission (“SEC”) and therefore do not include information or footnotes necessary for a complete presentation of financial position, results of operations and cash flows in conformity with accounting principles generally accepted in the United States of America. However, all adjustments (consisting only of normal recurring adjustments) which are, in the opinion of management, necessary for a fair presentation of the financial position and operating results for the periods presented have been included. These financial statements should be read in conjunction with the financial statements and notes thereto, together with Management’s Discussion and Analysis of Financial Condition and Results of Operations, contained in the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2008 (“10-K”), as filed with the SEC. The June 30, 2008 balance sheet was derived from audited financial statements, but does not include all disclosures required by accounting principles generally accepted in the United States of America. The results of operations for the six months ended December 31, 2008 are not necessarily indicative of the results for the full fiscal year ending June 30, 2009 or for any other period.
iBioPharma, Inc., a Delaware Corporation, (formerly InB:Biotechnologies, Inc., a New Jersey corporation) (the “Company”) and formerly a wholly owned subsidiary of Integrated BioPharma, Inc. (the “Former Parent” or “Integrated BioPharma”), is engaged primarily in the biotechnology business, which is focused on the discovery, development and commercialization of proprietary products from plants. The Company is developing its patented plant-based expression technologies for the production of vaccines, antibodies and other therapeutic proteins. The Company is also using plants as sources of novel, high quality nutritional supplements. The Company’s patented process for the hydroponic growth of edible plants causes them to accumulate high levels of important nutritional minerals. The Company’s customers are located primarily in the United States. The Company was incorporated on April 15, 1993 as Phytotech, Inc., subsequently changed its name to Nucycle Therapy, Inc. and in August 2008 was merged into iBioPharma, Inc., a newly formed Delaware Corporation, under its present name to effect a spin-off transaction.
On November 9, 2007, the Board of Directors of our Former Parent, approved a plan to distribute its equity interests in the Company to its stockholders. On July 25, 2008 our Former Parent announced the spin-off of the Company in the form of a dividend. The record date of the dividend was August 12, 2008 with a distribution date of August 18, 2008. Stockholders of our Former Parent received one share
of the Company’s common stock for each share of common stock they owned of our Former Parent as of the record date.
Immediately following the spin-off, the Company became a public company with stock traded on the OTC Bulletin Board under the symbol IBPM.OB.
The Company is operating in one business segment for all periods presented.
Our plans to expand our business and to continue to improve our product candidates to strengthen our ability to obtain licensees for our proprietary technology may require funds in excess of our cash flow and may require us to seek financing from third parties. In the past, Integrated BioPharma has provided capital for our general corporate purposes, and we used cash provided by Integrated BioPharma to fund our operations. Since the distribution, Integrated BioPharma has not and will not provide funds to finance our operations. Without the opportunity to obtain financing from Integrated BioPharma, we will in the future need to obtain additional financing from banks, or through public offerings or private placements of debt or equity securities, strategic relationships or other arrangements. The terms, interest rates, costs and fees of new credit facilities may not be as favorable as those historically enjoyed with Integrated BioPharma. For example, Integrated BioPharma did not charge us with any fees or costs for the intercompany borrowing, nor were there any covenants regarding financial ratios or prohibition on certain transactions in the loan arrangement with Integrated BioPharma. Our inability to obtain financing on favorable terms could restrict our operations and increase our losses.
In August 2008, we closed on our $5.0 million private placement, which funds were released from an escrow account subsequent to the spin-off. This additional capital is expected to cover our anticipated costs through the first quarter of calendar year 2010. If we are unsuccessful in raising additional capital or other alternative financing by then we might have to postpone or abandon our efforts to commercialize the intellectual property and suspend operations.
F-20
Note 2. Summary of Significant Accounting Policies
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
|
· |
sales returns and allowances; |
|
· |
allowance for doubtful accounts; |
|
· |
valuation and recoverability of long-lived and intangible assets, including the values assigned to acquired intangible assets; |
|
· |
income taxes and valuation allowance on deferred income taxes, and; |
|
· |
accruals for, and the probability of, the outcome of current litigation, if any. |
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Revenue Recognition. The Company recognizes revenue when the following four criteria under the Staff Accountant’s Bulletin (“SAB 104”) have been met: (i) persuasive evidence that an arrangement exists, (ii) the product has been shipped and the Company has no significant remaining obligation, (iii) the seller’s price to the buyer is fixed or determinable and (iv) collectability is reasonably assured. Among the factors the Company takes into account in determining the proper time at which to recognize revenue are when title of the goods transfers and when the risk of loss transfers. The Company’s sales policy is to require customers to provide purchase orders establishing selling prices and shipping terms. The Company evaluates the credit risk of each customer and establishes an allowance of doubtful accounts for any credit risk. Sales returns and allowances are estimated upon shipment.
Research and Development Costs. Research and development costs are expensed as incurred. The Company incurred $250,000 and $500,000 in the three and six months ended December 31, 2008, respectively with no research and development expenses incurred in the three and six months ended December 31, 2007.
Stock-Based Compensation. As of December 31, 2008, the Company has a stock-based compensation plan; however no shares have been issued under the Plan. Prior to the spin-off, non-cash compensation earned by employees and directors of the Company were the result of stock options and restricted stock unit awards issued under the Former Parent’s stock based compensation plan.
Income Taxes. The Company had elected to file its federal income tax return as part of the consolidated federal tax return of Integrated BioPharma, its then parent company, and accordingly has not filed separate tax returns with the Internal Revenue Service since it was a wholly owned subsidiary of Integrated BioPharma through
August 18, 2008. For state and local income taxes the Company has and continues to file tax returns separate from its Former Parent. Integrated BioPharma and the Company account for the Company’s federal tax liabilities on the “separate company basis” method in accordance with FASB Statement No. 109, “Accounting for Income Taxes”. Under this method, the Company records tax expense and related deferred tax benefits in a
manner comparable to that which it would record if it were not affiliated with Integrated BioPharma.
The Company will file separate federal tax returns beginning in its fiscal year ending June 30, 2009, which will be for the period from August 18, 2008 to June 30, 2009, subsequent filings will be for the Company’s entire fiscal year periods ending June 30.
The Company accounts for income taxes using the asset and liability method. Accordingly, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be
recovered or settled. The effect on deferred tax assets and liabilities of a change in the tax rate is recognized in income or expense in the period that the change is effective. Tax benefits are recognized when it is
F-21
probable that the deduction will be sustained. A valuation allowance is established when it is more likely than not that all or a portion of a deferred tax asset will either expire before the Company is able to realize the benefit, or that future deductibility is uncertain.
Earnings Per Share. In accordance with FASB Statement No. 128, “Earnings Per Share,” basic earnings per common share are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible preferred stock, subject to antidilution limitations.
For the three and six months ended December 31, 2008, the Company had warrants to purchase 2,345,752 shares of common stock outstanding that were not included in the computation of diluted earnings per share as their exercise prices were greater than the market price of the common shares as of December 31, 2008. For the three and six months ended December 31, 2007, the Company did not have any derivative securities outstanding which would result in the dilution of earnings per share.
Recent Accounting Pronouncements. In October 2008, the FASB issued FSP No. 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active”
(“FSP 157-3”). FSP 157-3 clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 was effective for us on December 31, 2008 for all financial assets and liabilities recognized or disclosed at fair value in our Condensed
Financial Statements on a recurring basis (at least annually).
In May 2008, the FASB issued SFAS No. 162, The Hierarchy of Generally Accepted Accounting Principles. The statement is intended to improve financial reporting by identifying a consistent hierarchy for selecting accounting principles to be used in preparing financial statements that are presented in conformity with GAAP. Prior to the issuance of SFAS No. 162, GAAP hierarchy was defined in the American Institute of Certified Public Accountants (“AICPA”) Statement on Auditing
Standards (SAS) No. 69, The Meaning of Present Fairly in Conformity With Generally Accepted Accounting Principles. Unlike SAS No. 69, SFAS No. 162 is directed to the entity rather than the auditor. Statement No. 162 is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board Auditing amendments to AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles. SFAS No. 162 is not expected to have any
material impact on the Company’s results of operations, financial condition or liquidity.
Note 3. Intangible Assets and Other Payables
The carrying amount of intangible assets as of December 31, 2008 and June 30, 2008 is as follows:
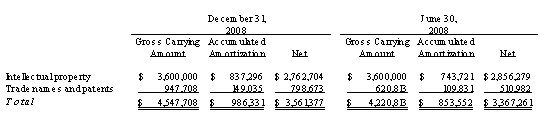
Intellectual property consists of exclusive licensing rights, patents and other technology relating to producing human health and veterinary influenza applications of the plant-based technology developed by the Center for Molecular Biotechnology of Fraunhofer USA, Inc. (“FhCMB”).
Under a Technology Transfer Agreement (the “TTA”) effective as of January 1, 2004, we acquired from FhCMB: (i) exclusive commercial rights to certain intellectual property invented and developed by FhCMB by which targeted proteins can be produced in plants for the development and manufacture of novel vaccines and therapeutics for humans and certain veterinary applications, and (ii) FhCMB’s commitment for maintenance and support services necessary to further protect the Platform, including filing and prosecuting patent applications, providing scientific support for patent counsel’s activities on behalf of the Company and otherwise to maintain in force and good standing the Company’s intellectual property rights. The total contract price for the Platform and the support and maintenance services was $3.0 million. In March 2006, and December 2007, the Company expanded the rights acquired from Fraunhofer to include veterinary and
F-22
diagnostic applications of the Platform, for $500,000 and $100,000, respectively, which increased the original purchase price from $3.0 million to $3.6 million.
The Company recorded the payments under the TTA and payments to patent counsel for protection of the Platform as intangible assets with a definite life using the payments made to determine the fair value of the intellectual properties acquired. The Company recorded the payments at the due dates provided in the TTA after knowing that Fraunhofer had provided the required maintenance and support services in that period. When the parties entered into the TTA, we expected the articulation and filing of U.S. patent and other intellectual property protections to be accomplished substantially evenly over the term of the TTA. However, by June 30, 2007, when the Company determined that substantially all of the maintenance and support activities had been performed in support of the Platform because all of the patents and foreign applications contemplated to be filed to protect the Platform had been completed, the Company booked the remainder of the payments due under the TTA.
During the six months ended December 31, 2008, the Company made the final payments of $1,050,000 under the intellectual property acquisition agreement, as amended, with FhCMB entered into in January 2004. As of June 30, 2008, the Company had the remaining commitment of $1,050,000 included in other payables. Amortization expense recorded on intangible assets for the three and six months ended December 31, 2008 and 2007 was approximately $69,100 and $56,200 and $132,800 and $125,700, respectively. Amortization expense is recorded on the straight-line method over periods ranging from 10 years to 20 years and is included in selling and administrative expenses.
The estimated annual amortization expense for intangible assets for the five succeeding fiscal years is as follows as of December 31, 2008:
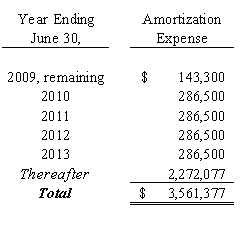
Note 4. Due to Former Parent and Other Transactions with Former Parent
Due to Former Parent consists of net cash advances from Integrated BioPharma to assist the Company in meeting its obligations and for corporate support charges, offset by our Former Parent’s use of the Company’s federal net operating loss. Integrated BioPharma did not charge the Company interest on any of these advances. These advances consisted of the following:
F-23
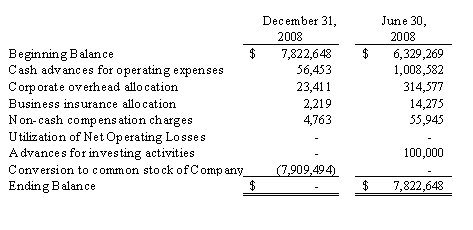
The corporate overhead allocation due our Former Parent was allocated based on the estimated time that Integrated BioPharma’s officers and employees dedicated to our Company’s business and included charges for employee salaries and benefits, legal, accounting and other consulting fees, treasury and tax services and general office expenses. The allocations were based on actual costs incurred by our Former Parent.
In August 2008, our Former Parent ceased allocating its corporate overhead to the Company and entered into a Transitional Services Agreement (the “TS Agreement”) with Integrated BioPharma. The transitional services agreement permits us to continue to use certain corporate services previously provided to us by Integrated BioPharma as a subsidiary corporation in exchange for a management charge. The scope of these services is limited to legal, strategic financial planning and SEC reporting, and tax services by certain Integrated BioPharma corporate employees. In exchange for these services, the Company expects to pay approximately $50,000 for certain financial and tax services over an estimated period of six months; the TS Agreement provides for a per annum fee of $100,000. In the three and six months ended December 31, 2008, Integrated BioPharma charged us approximately $25,000 and $37,000, respectively, under the TS Agreement.
Note 5. Significant Risks and Uncertainties
(a) Concentrations of Credit Risk-Cash. The Company maintains balances at a financial institution. Deposit accounts at the institution are insured by the Federal Deposit Insurance Corporation (the “FDIC”) for deposits up to $250,000. As of December 31, 2008, the Company had uninsured cash balances of approximately $1.6 million on deposit with JP Morgan Chase. The FDIC is temporarily insuring deposits up to $250,000 at financial institutions through December 31, 2009. Additionally, JP Morgan Chase is participating in the FDIC’s Transaction Account Guarantee Program, whereby all non-interest bearing checking accounts (including accounts with interest rates less than 0.50%) are fully guaranteed by the FDIC for the entire amount through December 31, 2009.
(b) Concentrations of Credit Risk-Receivables. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk of its customers, establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowances is limited. The Company does not require collateral in relation to its trade accounts receivable credit risk. The amount of the allowance for uncollectible accounts and other allowances as of December 31, 2008 and June 30, 2008 was $2,250. The Company’s bad debt expense for each of the three and six months ended December 31, 2008 and 2007 was none.
(c) Major Customers. For the three months ended December 31, 2008, approximately 12.1%, 39.8% and 43.1%, respectively and for the six months ended December 31, 2008, approximately 23.6%, 31.5% and 42.1%, respectively, of revenues were derived from three customers. For the three and six months ended December 31, 2007, approximately 58.8% and 39.1% and 48.8% and 49.4%, respectively, of revenues were derived from two customers. The loss of any of these customers would have an adverse affect on the Company’s sales. Accounts receivable from the three customers as of December 31, 2008, represents 90.5% of the accounts receivable balance as of such date.
(d) Major Supplier and Related Party. The Company has subcontracted the manufacturing, including the oversight of its supply agreement with a wholly owned subsidiary of Integrated BioPharma (IHT Health Products, Inc. (“IHT”)), which in turn contracts with another wholly owned subsidiary of Integrated BioPharma; substantially all of our cost of goods sold are paid to this related party. For the three and six months ended December 31, 2008 and 2007, the Company was invoiced by IHT $214,200 and $128,320 and $331,800 and $246,320, respectively under this arrangement which amounts are included in cost of
F-24
goods sold in the accompanying statements of operations and which are payable as and when payment is received by the Company from the sale of such goods. The Company is not direct billed by the other related party utilized under the manufacturing arrangement.
(e) Other Business Risks. The Company insures it business and assets against insurable risks, to the extent that it deems appropriate, based upon an analysis of the relative risks and costs. The Company believes that the risk of loss from non-insurable events would not have a material adverse effect on the Company’s operations as a whole.
Note 6. Commitments and Contingencies
(a) Leases. The Company leases office space on a month-to-month basis. The lease was effective October 1, 2006 and provides for a minimum monthly rental of $1,126. Total rent expense, including real estate taxes and maintenance charges, was approximately $3,400 and $6,800 for each of the three and six months ended December 31, 2008 and 2007, respectively.
(b) Intellectual Property and Research Agreements. In connection with the acquisition in January 2004 of intellectual property developed by the Center for Molecular Biotechnology of Fraunhofer USA, Inc. (“FhCMB”), the Company entered into a Technology Transfer Agreement on December 18, 2003 (the “IP Agreement”), whereby the Company agreed to pay up to a maximum of $3.0 million for
certain technology developed by FhCMB over a five-year period. In addition to the IP Agreement, the Company entered into research agreements, which require the payment of several milestone payments related to achieving certain flu vaccine studies and our ongoing Anthrax studies (the “R&D Agreements”).
In March, 2006, the Company amended their IP Agreement with FhCMB to expand the scope of the IP Agreement and increased the amount of the purchase commitment to a maximum of $3.5 million. In June 2007, the Company amended their existing amended IP Agreement and R&D Agreements with FhCMB, to commercialize the developed process, production techniques and methodologies of the proprietary technology and intellectual property for external applications. The June 2007 amendment requires
FhCMB to continue to conduct research to enhance, improve and expand the existing intellectual property, and for this research the Company has committed to make non-refundable payments of $2.0 million per year for five years, aggregating to $10.0 million, beginning in November 2009. In addition, the Company will make royalty payments to FhCMB based on receipts derived by the Company from sales of products utilizing the proprietary technology for a period of fifteen years instead of the
original the ten-year period. In turn, FhCMB shall pay the Company royalty payments for all receipts, if any, realized by FhCMB sales, licensing or commercialization of the intellectual property acquired by them for the same fifteen-year period. Furthermore, FhCMB has agreed to expend at a minimum, an additional $2.0 million per year in the same timeframe as the Company for research and development on the intellectual property.
In December 2007, the Company and FhCMB further amended the IP Agreement increasing the purchase price by $100,000 to amend the field to include influenza diagnostics for a maximum purchase price of $3.6 million.
As of December 31, 2008, the Company has made payments in full for the purchase commitment of $3.6 million.
Note 7. Equity Transactions
In November 2007, the Company entered into a Separation and Distribution Agreement (the “Distribution”) with its Former Parent, whereby, the Former Parent agreed to distribute, pro rata, to the holders of its common stock, all of the shares it owned of the Company’s common stock. The completion of the Distribution was subject to various customary closing conditions, including the declaration by the U.S. Securities and Exchange Commission of the effectiveness of the registration under the Securities Exchange Act of 1934 of the Company’s common stock. The Distribution was completed on August 18, 2008 and each shareholder of our Former Parent received one share of the Company for each share the shareholder owned as of August 12, 2008, the Record Date. The Distribution should qualify as a tax-free reorganization under Section 355 of the Internal Revenue Code of 1986, as amended. The Separation and Distribution Agreement prohibits the Company from issuing more than 19,845,061 of additional shares of its common stock (representing the number of shares issued in connection with the Distribution) for the two years immediately following the effective date of the Distribution.
F-25
Additionally, on August 19, 2008, our Former Parent entered into a Conversion Agreement, whereby Integrated BioPharma caused approximately $5.2 million of the intercompany debt to be contributed to additional paid in capital and used $2.7 million of the intercompany debt to purchase approximately 1.3 million shares of the Company, representing 6% of the then outstanding shares of the Company. Subsequent to the Company’s private placement as discussed below, Integrated BioPharma
owns 5.4% of the Company.
Also, on August 19, 2008, the Company closed on its $5.0 million capital raise in connection with its private placement of approximately ten percent (10%) of the Company, such funds were released to the Company from the escrow and issued approximately 2.3 million shares of the Company’s par value $0.001 common stock, at an estimated purchase price of approximately $2.13 per share. The Company’s net proceeds from its private placement were approximately $4.6 million after payment of certain expenses related to the capital raise.
The Company also issued to the private placement investors, warrants to purchase a number of shares of common stock equal to 50% of the number of shares purchased by such private placement investor, with an exercise price equal to 150% of the purchase price of the Company’s common stock subject to adjustments therein and warrants to purchase a number of shares of common stock equal to 50% of the number of shares purchased by such private placement investor, with an exercise price equal to 200% of the purchase price of the Company’s common stock subject to adjustments therein and exercisable over the next five-year period.
F-26
4,691,504 Shares of Common Stock
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the various expenses that will be paid by us in connection with the securities being registered. With the exception of the SEC registration fee, all amounts shown are estimates:
|
|
$ |
528.81 |
|
|
|
Federal Taxes |
|
|
-- |
|
|
State Taxes |
|
|
-- |
|
|
Legal Fees and Expenses |
|
|
35,000 |
|
|
Printing and Engraving Expenses |
|
|
-- |
|
|
Blue Sky Fees |
|
|
-- |
|
|
Accounting Fees and Expenses |
|
|
5,000 |
|
|
Miscellaneous |
|
|
471.19 |
|
|
Total |
|
$ |
41,000 |
We will pay all expenses incurred in connection with the registration of the shares covered by this prospectus. Brokerage commissions, underwriters’ fees, discounts and commissions and similar selling expenses, if any, attributable to the sale of the shares covered by this prospectus will be borne by the selling stockholders.
Item 14. Indemnification of Directors and Officers.
Our Certificate of Incorporation will provide for indemnification of our officers and directors to the extent permitted by Delaware law, which generally permits indemnification for actions taken by officers or directors as our representatives if the officer or director acted in good faith and in a manner he or she reasonably believed to be in the best interest of the corporation. We have entered into indemnification agreements with our officers and directors to specify the terms of our indemnification obligations. In general, these indemnification agreements provide that we will:
|
· |
indemnify our directors and officers to the fullest extent now permitted under current law and to the extent the law later is amended to increase the scope of permitted indemnification; |
|
· |
advance payment of expenses to a director or officer incurred in connection with an indemnifiable claim, subject to repayment if it is later determined that the director or officer was not entitled to be indemnified; |
|
· |
reimburse the director or officer for any expenses incurred by the director or officer in seeking to enforce the indemnification agreement; and |
|
· |
have the opportunity to participate in the defense of any indemnifiable claims against the director or officer. |
As permitted under Delaware law, the By-laws contain a provision indemnifying directors against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by them in connection with an action, suit or proceeding if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of our Company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe their conduct was unlawful.
The separation and distribution agreement that we have entered into with Integrated BioPharma provides for indemnification by us of Integrated BioPharma and its directors, officers and employees for some liabilities, including liabilities under the Securities Act and the Securities Exchange Act of 1934 in connection with the distribution, and a mutual indemnification of each other for product liability claims arising from their respective businesses, and also requires that we indemnify Integrated BioPharma for various liabilities of iBioPharma, and for any tax that may be imposed with respect to the distribution and which result from our actions or omissions in that regard.
Item 15. Recent Sales of Unregistered Securities
II-1
None.
Item 16. Exhibits
Exhibits filed with this Registration Statement on Form S-1 or incorporated by reference from other filings are as follows:
|
Number |
Description |
|
|
|
|
3.1 |
Form of Articles of Incorporation of iBioPharma, Inc. (3) |
|
3.2 |
Form of Bylaws of iBioPharma, Inc. (3) |
|
4.1 |
Form of Common Stock Certificate (3) |
|
4.2 |
Form of Warrant to Purchase Common Stock of iBioPharma, Inc. for each Investor (5) |
|
5.1 |
Opinion of Davis Wright Tremaine LLP (8) |
|
10.1 |
Separation and Distribution Agreement, dated as of November 14, 2007, between Integrated BioPharma, Inc. and the Registrant. (1) |
|
10.2 |
Indemnification and Insurance Matters Agreement between Integrated BioPharma, Inc., and the Registrant (5) |
|
10.3 |
Transitional Services Agreement between Integrated BioPharma, Inc. and the Registrant. (5) |
|
10.4 |
Tax Allocation Agreement between Integrated BioPharma, Inc. and the Registrant. (5) |
|
10.5 |
Form of Securities Purchase Agreement between various purchasers and the Registrant. (6) |
|
10.6 |
Technology Transfer Agreement, dated as of January 1, 2004, between the Registrant and Fraunhofer USA Center for Molecular Biotechnology, Inc. (3) |
|
10.7 |
Non-Standard Navy Cooperative Research and Development Agreement, dated August 17, 2004, between the Registrant and Fraunhofer USA Center for Molecular Biotechnology, Inc. (2) |
|
10.8 |
Supply License Agreement, dated as of March 22, 2006, between the Registrant and Mannatech, Inc. (2) |
|
10.9 |
Form of Registration Rights Agreement with iBioPharma, Inc. for each Investor. (6) |
|
10.10 |
Conversion Agreement, dated August 19, 2008, by and between iBioPharma, Inc. and Integrated BioPharma, Inc. (6) |
|
10.11 |
2008 Omnibus Equity Compensation Plan of iBioPharma, Inc. (8) |
|
21.1 |
Subsidiaries of the Registrant (7) |
|
23.1 |
Consent of Amper, Politziner & Mattia, LLP (8) |
|
23.2 |
Consent of Davis Wright Tremaine LLP (included in Exhibit 5.1) |
|
24.1 |
Power of Attorney (included in the signature page of this Registration Statement) |
__________________________
|
(1) |
Incorporated herein by reference to the Company’s Form 10-12G filed with the Commission on March 7, 2008. |
|
(2) |
Incorporated herein by reference to the Company’s Form 10-12G filed with the Commission on June 18, 2008. |
|
(3) |
Incorporated herein by reference to the Company’s Form 10-12G filed with the Commission on July 11, 2008. |
|
(4) |
Incorporated herein by reference to the Company’s Form 10-12G filed with the Commission on July 17, 2008. |
|
(5) |
Incorporated herein by reference to the Company’s Current Report on Form 8-K filed with the SEC on August 12, 2008. |
|
(6) |
Incorporated herein by reference to the Company’s Current Report on Form 8-K filed with the SEC on August 19, 2008. |
|
(7) |
Incorporated herein by reference to the Company’s Annual Report on Form 10-K for the year ended June 30, 2008. |
|
(8) |
Filed herewith. |
Item 17. Undertakings.
(a) We hereby undertake:
(1) to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
(i) |
to include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
II-2
|
(ii) |
to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
|
(iii) |
to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
(2) that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
(3) to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and
Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the
securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, iBioPharma, Inc. has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Newark, Delaware, on April 24, 2009.
IBIOPHARMA, INC.
By: /s/ Robert B. Kay
Robert B. Kay
Chief Executive Officer
POWER OF ATTORNEY
We, the undersigned officers and directors of the Registrant, iBioPharma, Inc., a Delaware corporation, hereby severally and individually constitute and appoint Robert B. Kay, Chief Executive Officer and Dina L. Masi, Interim Chief Financial Officer, and each of them, as true and lawful attorneys in fact for the undersigned, in any and all capacities, with
full power of substitution, to sign any and all amendments to this Registration Statement (including post-effective amendments), and to file the same with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys in fact, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as
he or she might or could do in person, hereby ratifying and confirming all that said attorneys in fact, or any of them, may lawfully do or cause to be done by virtue of this appointment.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
II-4