
Filed Pursuant to Rule 424(b)(3)
File Number 333-158759
PROSPECTUS SUPPLEMENT NO. 1
Prospectus Supplement No. 1
to Prospectus dated May 13, 2009
IBIOPHARMA, INC.
This Prospectus Supplement No. 1 supplements our Prospectus dated May 13, 2009. The shares that are the subject of the Prospectus have been registered to permit their resale to the public by the selling stockholders named in the Prospectus. We are not selling any shares of common stock in this offering and therefore will not receive any proceeds from this offering, except upon the exercise of warrants.
Our common stock is quoted on the OTC Bulletin under the symbol IBPM.OB. On May 15, 2009, the closing price of our common stock on the OTC Bulletin Board was $0.40.
This Prospectus Supplement includes the following attached item: Quarterly Report on Form 10-Q for the quarter ended March 31, 2009, as filed by us with the Commission on May 15, 2009.
YOU SHOULD READ THE PROSPECTUS AND THIS PROSPECTUS SUPPLEMENT NO. 1, INCLUDING THE RISK FACTORS THAT BEGIN ON PAGE 3 OF THE PROSPECTUS.
NEITHER THE U.S. SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS SUPPLEMENT IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this Prospectus Supplement is May 18, 2009.
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 10-Q
X Quarterly Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the quarterly period ended March 31, 2009
OR
Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from to
Commission File Number 000-53125
iBioPharma, Inc.
(Exact name of small business registrant in its charter)
|
Delaware |
|
26-2797813 |
|
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
|
9 Innovation Way, Suite 100, Newark, DE |
|
19711 |
|
|
(Address of principal executive offices) |
|
(Zip Code) |
(302) 355-0650
(Registrant’s telephone number, including Area Code)
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
|
Yes þ |
No o |
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
|
|
|
|
|
|
|
|
Large accelerated filer o |
|
Accelerated filer o |
|
Non-accelerated filer o |
|
Smaller reporting company þ |
Indicate by check whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
|
Yes o |
No þ |
The number of shares outstanding of each of the issuer’s class of common stock, as of the latest practicable date:
|
Class |
|
Outstanding at May 15, 2009 |
|
|
Common Stock, $0.001 par value |
|
23,357,519 Shares |
IBIOPHARMA, INC.
FORM 10-Q QUARTERLY REPORT
For the Three and Nine Months Ended March 31, 2009
INDEX
|
|
|
Page |
|
|
Part I. Financial Information |
|
|
Item 1. |
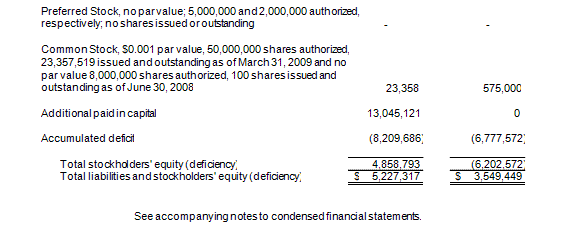
Condensed Balance Sheets as of March 31, 2009 (unaudited) and June 30, 2008 |
2 |
|
|
Condensed Statements of Operations for The Three and Nine Months Ended March 31, 2009 and 2008 (unaudited) |
3 |
|
|
Condensed Statements of Changes in Stockholders’ Equity (Deficiency) for The Nine Months Ended March 31, 2009 (unaudited) |
4 |
|
|
Condensed Statements of Cash Flows for The Nine Months Ended March 31, 2009 and 2008 (unaudited) |
5 |
|
|
Notes to Condensed Financial Statements |
6 |
|
|
|
|
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
16 |
|
|
|
|
|
Item 3. |
Quantitative and Qualitative Disclosures about Market Risk |
28 |
|
|
|
|
|
Item 4T. |
Controls and Procedures |
28 |
|
|
|
|
|
|
Part II. Other Information |
|
|
|
|
|
|
Item 1. |
Legal Proceedings |
29 |
|
|
|
|
|
Item 1A. |
Risk Factors |
29 |
|
|
|
|
|
Item 2. |
Unregistered Sales of Equity Securities and Use of Proceeds |
29 |
|
|
|
|
|
Item 3. |
Defaults Upon Senior Securities |
29 |
|
|
|
|
|
Item 4. |
Submission of Matters to a Vote of Security Holders |
29 |
|
|
|
|
|
Item 5. |
Other Information |
29 |
|
|
|
|
|
Item 6. |
Exhibits |
30 |
|
|
Other |
|
|
Signatures |
|
31 |
Disclosure Regarding Forward-Looking Statements
Certain statements in the Quarterly Report on Form 10-Q may constitute “forward-looking” statements as defined in Section 27A of the Securities Act of 1933 (the “Securities Act”), Section 21E of the Securities Act of 1934 (the “Exchange Act”), the Private Securities Litigation Reform Act of
1995 (the “PSLRA”) or in releases made by the Securities and Exchange Commission, all as may be amended from time to time. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of iBioPharma, Inc. or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Statements that
are not historical fact are forward-looking statements. Forward-looking statements can be identified by, among other things, the use of forward-looking language, such as the words, “plan”, “believe”, “expect”, “anticipate”, “intend”, “estimate”, “project”, “may”, “will”, “would”, “could”, “should”, “seeks”, or “scheduled
to”, or other similar words, or the negative of these terms or other variations of these terms or comparable language, or by discussion of strategy or intentions. These cautionary statements are being made pursuant to the Securities Act, the Exchange Act and the PSLRA with the intention of obtaining the benefits of the “safe harbor” provisions of such laws.
The Company cautions investors that any forward-looking statements made by the Company are not guarantees or indicative of future performance. Important assumptions and other important factors that could cause actual results to differ materially from those forward-looking statements with respect to the Company, include, but are
not limited to, the risks and uncertainties affecting its businesses described in Item 1 of the Company’s Annual Report filed on Form 10-K for the year ended June 30, 2008 and in registration statements and other securities filings by the Company. Although the Company believes that its plans, intentions and expectations reflected in or suggested by such forward-looking statements are reasonable, actual results
could differ materially from a projection or assumption in any of its forward-looking statements, are subject to change and inherent risks and uncertainties.
The forward-looking statements contained in this Quarterly Report on Form 10-Q are made only as of the date hereof and the Company does not have or undertake any obligation to update or revise any forward-looking statements whether as a result of new information, subsequent events or otherwise, unless otherwise required by
law.
ITEM 1. FINANCIAL STATEMENTS


Page 2
Page 3
Page 4
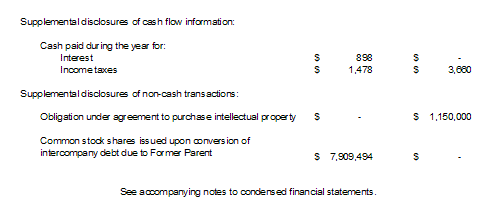
Page 5
iBioPharma, Inc.
(Formerly, InB:Biotechnologies, Inc.)
Notes To Condensed Financial Statements
(Unaudited)
Note 1. Principles of Consolidation and Basis of Presentation and Liquidity
The accompanying financial statements for the interim periods are unaudited and include the accounts of the Company. The interim financial statements have been prepared in conformity with Rule 10-01 of Regulation S-X of the Securities and Exchange Commission (“SEC”) and therefore
do not include information or footnotes necessary for a complete presentation of financial position, results of operations and cash flows in conformity with accounting principles generally accepted in the United States of America. However, all adjustments (consisting only of normal recurring adjustments) which are, in the opinion of management, necessary for a fair presentation of the financial position and operating results for the periods presented have been included. These financial
statements should be read in conjunction with the financial statements and notes thereto, together with Management’s Discussion and Analysis of Financial Condition and Results of Operations, contained in the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2008 (“10-K”), as filed with the SEC. The June 30, 2008 balance sheet was derived from audited financial statements, but the related footnotes do
not include all disclosures required by accounting principles generally accepted in the United States of America. The results of operations for the nine months ended March 31, 2009 are not necessarily indicative of the results for the full fiscal year ending June 30, 2009 or for any other period.
iBioPharma, Inc., a Delaware Corporation, (formerly InB:Biotechnologies, Inc., a New Jersey corporation) (the “Company”) and formerly a wholly owned subsidiary of Integrated BioPharma, Inc. (the “Former Parent” or “Integrated BioPharma”), is engaged primarily in the biotechnology business, which is focused on the discovery, development and commercialization of proprietary products from plants. The Company is developing its patented plant-based expression technologies for the production of vaccines, antibodies and other therapeutic proteins. The Company is also using plants as sources of novel, high quality nutritional supplements. The Company’s patented process for the hydroponic growth of edible plants causes them to accumulate high levels of important nutritional minerals. The Company’s customers are located primarily in the United States. The Company was incorporated on April 15, 1993 as Phytotech, Inc., subsequently changed its name to Nucycle Therapy, Inc. and in August 2008 was merged into iBioPharma, Inc., a newly formed Delaware Corporation, under its present name to effect a spin-off transaction.
On November 9, 2007, the Board of Directors of our Former Parent, approved a plan to distribute its equity interests in the Company to its stockholders. On July 25, 2008 our Former Parent announced the spin-off of the Company in the form of a dividend. The record date of the dividend was August 12, 2008 with a distribution date of August 18, 2008. Stockholders of our Former Parent received one share of the Company’s common stock for each share of common stock they owned of our Former Parent as of the record date.
Page 6
Immediately following the spin-off, the Company became a public company with stock traded on the OTC Bulletin Board under the symbol IBPM.OB.
The Company is operating in one business segment for all periods presented.
Our plans to expand our business and to continue to improve our product candidates to strengthen our ability to obtain licensees for our proprietary technology may require funds in excess of our cash flow and may require us to seek financing from third parties. In the past, Integrated BioPharma has provided capital for our general corporate purposes, and we used cash provided by Integrated BioPharma to fund our operations. Since the distribution, Integrated BioPharma has not and will
not provide funds to finance our operations. Without the opportunity to obtain financing from Integrated BioPharma, we will in the future need to obtain additional financing from banks, or through public offerings or private placements of debt or equity securities, strategic relationships or other arrangements. The terms, interest rates, costs and fees of new credit facilities may not be as favorable as those historically enjoyed with Integrated BioPharma. For example, Integrated
BioPharma did not charge us with any fees or costs for the intercompany borrowing, nor were there any covenants regarding financial ratios or prohibition on certain transactions in the loan arrangement with Integrated BioPharma. Our inability to obtain financing on favorable terms could restrict our operations and increase our losses.
In August 2008, we closed on our $5.0 million private placement, which funds were released from an escrow account subsequent to the spin-off. This additional capital is expected to cover our anticipated costs through the first quarter of calendar year 2010. If we are unsuccessful in raising additional capital or other alternative financing by then we might have to postpone or abandon our efforts to commercialize the intellectual property and suspend operations.
Note 2. Summary of Significant Accounting Policies
Use of Estimates.
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. The most significant estimates include:
|
· |
sales returns and allowances; |
|
· |
allowance for doubtful accounts; |
|
· |
valuation and recoverability of long-lived and intangible assets, including the values |
Page 7
|
|
assigned to acquired intangible assets; |
|
· |
income taxes and valuation allowance on deferred income taxes, and; |
|
· |
accruals for, and the probability of, the outcome of current litigation, if any. |
On a continual basis, management reviews its estimates utilizing currently available information, changes in facts and circumstances, historical experience and reasonable assumptions. After such reviews, and if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates.
Revenue Recognition.
The Company recognizes revenue when the following four criteria under the Staff Accountant’s Bulletin (“SAB 104”) have been met: (i) persuasive evidence that an arrangement exists, (ii) the product has been shipped and the Company has no significant remaining obligation, (iii) the seller’s price to the buyer is fixed or determinable and (iv) collectability is reasonably assured. Among the factors the Company takes into account in determining the proper time at which to recognize revenue are when title of the goods transfers and when the risk of loss transfers. The Company’s sales policy is to require customers to provide purchase orders establishing selling prices and shipping terms. The Company evaluates the credit risk of each customer and establishes an allowance of doubtful accounts for any credit risk. Sales returns and allowances are estimated upon shipment.
Research and Development Costs.
Research and development costs are expensed as incurred. The Company incurred $83,100 and $714,300 in the three and nine months ended March 31, 2009, respectively, and $257,200 and $257,200 in the three and nine months ended March 31, 2008, respectively.
Stock-Based Compensation.
The Company records stock based compensation in accordance with Statement of Financial Accounting Standards No. 123(R), Share Based Payment ("FAS 123(R)") which requires that stock-based compensation expense be recognized in the financial statements for share based payment transactions and that the measurement of such expense be based upon the estimated fair value of the financial instruments issued.
Prior to the spin-off, non-cash compensation earned by employees and directors of the Company were the result of stock options and restricted stock unit awards issued under the Former Parent’s stock based compensation plan.
Page 8
Income Taxes.
The Company had elected to file its federal income tax return as part of the consolidated federal tax return of Integrated BioPharma, its then parent company, and accordingly has not filed separate tax returns with the
Internal Revenue Service since it was a wholly owned subsidiary of Integrated BioPharma through August 18, 2008. For state and local income taxes the Company has and continues to file tax returns separate from its Former Parent. Integrated BioPharma and the Company account for the Company’s federal tax liabilities on the “separate company basis” method in accordance with FASB Statement No. 109, “Accounting for Income Taxes”. Under this method, the Company
records tax expense and related deferred tax benefits in a manner comparable to that which it would record if it were not affiliated with Integrated BioPharma.
The Company will file separate federal tax returns beginning in its fiscal year ending June 30, 2009, which will be for the period from August 18, 2008 to June 30, 2009, subsequent filings will be for the Company’s fiscal year periods ending each June 30th.
The Company accounts for income taxes using the asset and liability method. Accordingly, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in the tax rate is recognized in income or expense in the period that the change is effective. Tax benefits are recognized when it is probable that the deduction will be sustained. A valuation allowance is established when it is more likely than not that all or a portion of a deferred tax asset will either expire before the Company is able to realize the benefit, or that future deductibility is uncertain.
Earnings Per Share.
In accordance with FASB Statement No. 128, “Earnings Per Share,” basic earnings per common share are based on weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible preferred stock, subject to antidilution limitations.
Diluted weighted-average shares are the same as basic weighted-average shares since the inclusion of potentially issuable shares pursuant to the exercise of stock options and warrants would have been antidilutive. The Company had options and warrants outstanding as of March 31, 2009 for the purchase of 1,080,000 and 2,345,752 common stock shares, respectively. There were no security instruments outstanding as of March 31, 2008 which could potentially result in the dilution of earnings per share.
Page 9
Reclassifcations.
Certain amounts of prior periods have been reclassified in these condensed financial statements in order to conform to the current period presentation.
Recent Accounting Pronouncements.
In October 2008, the FASB issued FSP No. 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active” (“FSP 157-3”). FSP 157-3 clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 was effective for us on December 31, 2008 for all financial assets and liabilities recognized or disclosed at fair value in our Condensed Financial Statements on a recurring basis (at least annually).
In June 2008, the FASB ratified the consensus reached on Emerging Issues Task Force ("EITF") Issue No. 07-05, Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity's Own Stock ("EITF No. 07-05"). EITF No. 07-05 clarifies the determination of whether an instrument (or an embedded feature) is indexed to an entity's own stock, which would qualify as a scope exception under SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities. EITF No. 07-05 is effective for financial statements issued for fiscal years beginning after December 15, 2008. The Company is currently evaluating the impact of the adoption of this pronouncement will have upon its financial statements.
In May 2008, the FASB issued FASB Staff Position (FSP) APB 14-1, “Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)” (FSP APB 14-1). FSP APB 14-1 clarifies that convertible debt instruments that may be settled in cash upon either mandatory or optional conversion (including partial cash settlement) are not addressed by paragraph 12 of APB Opinion No. 14, “Accounting for Convertible Debt and Debt issued with Stock Purchase Warrants.” Additionally, FSP APB 14-1 specifies that issuers of such instruments should separately account for the liability and equity components in a manner that will reflect the entity’s nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. FSP APB 14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. The Company is evaluating the impact the adoption of this pronouncement will have on its financial statements.
In May 2008, the FASB issued SFAS No. 162, The Hierarchy of Generally Accepted Accounting Principles. The statement is intended to improve financial reporting by identifying a consistent hierarchy for selecting accounting principles to be used in preparing financial statements that are presented in conformity with GAAP. Prior to the issuance of SFAS No. 162, GAAP hierarchy was defined in the American Institute of Certified Public Accountants (“AICPA”) Statement on Auditing Standards (SAS) No. 69, The Meaning of Present Fairly in Conformity With Generally Accepted Accounting Principles. Unlike SAS No. 69, SFAS No. 162 is directed to the entity rather than the auditor. Statement No. 162 is effective 60 days following
Page 10
the SEC’s approval of the Public Company Accounting Oversight Board Auditing amendments to AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles. SFAS No. 162 is not expected to have any material impact on the Company’s results of operations, financial condition or liquidity.
Note 3. Intangible Assets and Other Payables
The carrying amount of intangible assets as of March 31, 2009 and June 30, 2008 is as follows:
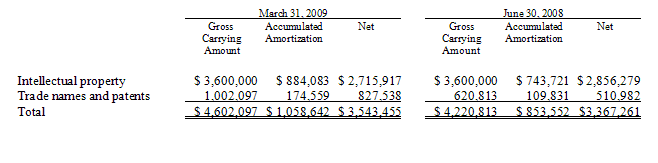
Intellectual property consists of exclusive licensing rights, patents and other technology relating to producing human health and veterinary influenza applications of the plant-based technology developed by the Center for Molecular Biotechnology of Fraunhofer USA, Inc. (“FhCMB”).
Under a Technology Transfer Agreement (the “TTA”) effective as of January 1, 2004, we acquired from FhCMB: (i) exclusive commercial rights to certain intellectual property invented and developed by FhCMB by which targeted proteins can be produced in plants for the
development and manufacture of novel vaccines and therapeutics for humans and certain veterinary applications, and (ii) FhCMB’s commitment for maintenance and support services necessary to further protect the Platform, including filing and prosecuting patent applications, providing scientific support for patent counsel’s activities on behalf of the Company and otherwise to maintain in force and good standing the Company’s intellectual property rights. The total
contract price for the Platform and the support and maintenance services was $3.0 million. In March 2006, and December 2007, the Company expanded the rights acquired from Fraunhofer to include veterinary and diagnostic applications of the Platform, for $500,000 and $100,000, respectively, which increased the original purchase price from $3.0 million to $3.6 million.
The Company recorded the payments under the TTA and payments to patent counsel for protection of the Platform as intangible assets with a definite life using the payments made to determine the fair value of the intellectual properties acquired. The Company recorded the payments at the due dates provided in the TTA after knowing that Fraunhofer had provided the required maintenance and support services in that period. When the parties entered into the TTA, we expected the articulation and filing of U.S. patent and other intellectual property protections to be accomplished substantially evenly over the term of the TTA. However, by June 30, 2007, when the Company determined that substantially all of the maintenance and support activities had been performed in support of the Platform because all of the patents and foreign applications contemplated to be filed to protect the Platform had been completed, the Company booked the remainder of the payments due under the TTA.
Page 11
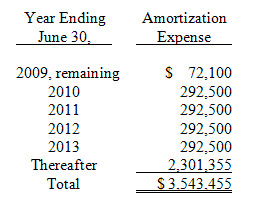
During the nine months ended March 31, 2009, the Company made the final payments of $1,050,000 under the intellectual property acquisition agreement, as amended, with FhCMB entered into in January 2004. As of June 30, 2008, the Company had the remaining commitment of $1,050,000 included in other payables. Amortization expense recorded on intangible assets for the three and nine months ended March 31, 2009 and 2008 was approximately $72,300 and $59,300 and $205,100 and $185,000, respectively. Amortization expense is recorded on the straight-line method over periods ranging from 10 years to 20 years and is included in selling and administrative expenses.
The estimated annual amortization expense for intangible assets for the five succeeding fiscal years is as follows as of March 31, 2009:
Note 4. Due to Former Parent and Other Transactions with Former Parent
Due to Former Parent consists of net cash advances from Integrated BioPharma to assist the Company in meeting its obligations and for corporate support charges, offset by our Former Parent’s use of the Company’s federal net operating loss. Integrated BioPharma did not charge the Company interest on any of these advances. These advances consisted of the following:
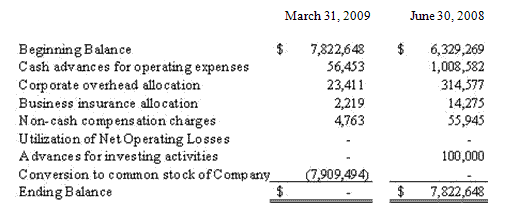
The corporate overhead allocation due our Former Parent was allocated based on the estimated time that Integrated BioPharma’s officers and employees dedicated to our Company’s business and included charges for employee salaries and benefits, legal, accounting and other consulting
Page 12
fees, treasury and tax services and general office expenses. The allocations were based on actual costs incurred by our Former Parent.
In August 2008, our Former Parent ceased allocating its corporate overhead to the Company and entered into a Transitional Services Agreement (the “TS Agreement”) with Integrated BioPharma. The transitional services agreement permits us to continue to use certain corporate services previously provided to us by Integrated BioPharma as a subsidiary corporation in exchange for a management charge. The scope of these services is limited to legal, strategic financial planning and SEC reporting, and tax services by certain Integrated BioPharma corporate employees. In exchange for these services, the Company expects to pay approximately $75,000 for certain financial and tax services over an estimated period of nine months; the TS Agreement provides for a per annum fee of $100,000. In the three and nine months ended March 31, 2009, Integrated BioPharma charged us approximately $25,000 and $62,000, respectively, under the TS Agreement.
Note 5. Significant Risks and Uncertainties
(a) Concentrations of Credit Risk-Cash.
The Company maintains balances at a financial institution. Deposit accounts at the institution are insured by the Federal Deposit Insurance Corporation (the “FDIC”) for deposits up to $250,000. As of March 31, 2009, the Company had cash balances of approximately $1.4
million on deposit with JP Morgan Chase which were insured by the FDIC.
The FDIC is temporarily insuring deposits up to $250,000 at financial institutions through December 31, 2009. Additionally, JP Morgan Chase is participating in the FDIC’s Transaction Account Guarantee Program, whereby all non-interest bearing checking accounts (including accounts with interest rates less than 0.50%) are fully guaranteed by the FDIC for the entire amount through December 31, 2009.
(b) Concentrations of Credit Risk-Receivables.
The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk of its customers, establishes an allowance for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowances is limited. The Company does not require collateral in relation to its trade accounts receivable credit risk. The amount of the allowance for uncollectible accounts and other allowances as of March 31, 2009 and June 30, 2008 was zero and $2,250, respectively. The Company had no bad debt expense for each of the three and nine month periods ended March 31, 2009 and 2008.
(c) Major Customers.
For the three months ended March 31, 2009, approximately 0%, 44% and 58%, respectively and for the nine months ended March 31, 2009, approximately 16%, 43% and 40%, respectively, of revenues were derived from three customers. For the three and nine months ended March 31, 2008, approximately 40% and 53% and 45% and 50%, respectively, of revenues were derived from two customers. The loss of any of these customers would have an adverse affect on the Company’s sales. Accounts receivable from the two customers as of March 31,
Page 13
2009, represents 92% of the accounts receivable balance as of such date.
(d) Major Supplier and Related Party.
The Company has subcontracted the manufacturing, including the oversight of its supply agreement with a wholly owned subsidiary of Integrated BioPharma (IHT Health Products, Inc. (“IHT”)), which in turn contracts with another wholly owned subsidiary of Integrated BioPharma; substantially all of our cost of goods sold are paid to this related party. For the three and nine months ended March 31, 2009 and 2008, the Company was invoiced by IHT $170,000 and $192,000 and $501,800 and $438,300, respectively, under this arrangement which amounts are included in cost of goods sold in the accompanying statements of operations and which are payable as and when payment is received by the Company from the sale of such goods. The Company is not direct billed by the other related party utilized under the manufacturing arrangement.
(e) Other Business Risks.
The Company insures it business and assets against insurable risks, to the extent that it deems appropriate, based upon an analysis of the relative risks and costs. The Company believes that the risk of loss from non-insurable events would not have a material adverse effect on the Company’s operations as a whole.
Note 6. Commitments and Contingencies
(a) Leases.
The Company leases office space on a month-to-month basis. The lease was effective October 1, 2006 and provides for a minimum monthly rental of $1,126. Total rent expense, including real estate taxes and maintenance charges, was approximately $3,400 and $10,100 for each of the three and nine months ended March 31, 2009 and 2008, respectively.
(b) Intellectual Property and Research Agreements.
In connection with the acquisition in January 2004 of intellectual property developed by the Center for Molecular Biotechnology of Fraunhofer USA, Inc. (“FhCMB”), the Company entered into a Technology Transfer Agreement on December 18, 2003 (the “IP Agreement”), whereby the Company agreed to pay up to a maximum of $3.0 million for certain technology developed by FhCMB over a five-year period. In addition to the IP Agreement, the Company entered into research agreements, which require the payment of several milestone payments related to achieving certain flu vaccine studies and our ongoing Anthrax studies (the “R&D Agreements”).
Page 14
In March, 2006, the Company amended their IP Agreement with FhCMB to expand the scope of the IP Agreement and increased the amount of the purchase commitment to a maximum of $3.5 million. In June 2007, the Company amended their existing amended IP Agreement and R&D Agreements with FhCMB,
to commercialize the developed process, production techniques and methodologies of the proprietary technology and intellectual property for external applications. The June 2007 amendment requires FhCMB to continue to conduct research to enhance, improve and expand the existing intellectual property, and for this research the Company has committed to make non-refundable payments of $2.0 million per year for five years, aggregating to $10.0 million, beginning in November 2009. In
addition, the Company will make royalty payments to FhCMB based on receipts derived by the Company from sales of products utilizing the proprietary technology for a period of fifteen years instead of the original the ten-year period. In turn, FhCMB shall pay the Company royalty payments for all receipts, if any, realized by FhCMB sales, licensing or commercialization of the intellectual property acquired by them for the same fifteen-year period. Furthermore, FhCMB has agreed to expend
at a minimum, an additional $2.0 million per year in the same timeframe as the Company for research and development on the intellectual property.
In December 2007, the Company and FhCMB further amended the IP Agreement increasing the purchase price by $100,000 to amend the field to include influenza diagnostics for a maximum purchase price of $3.6 million.
As of March 31, 2009, the Company has made payments in full for the purchase commitment of $3.6 million.
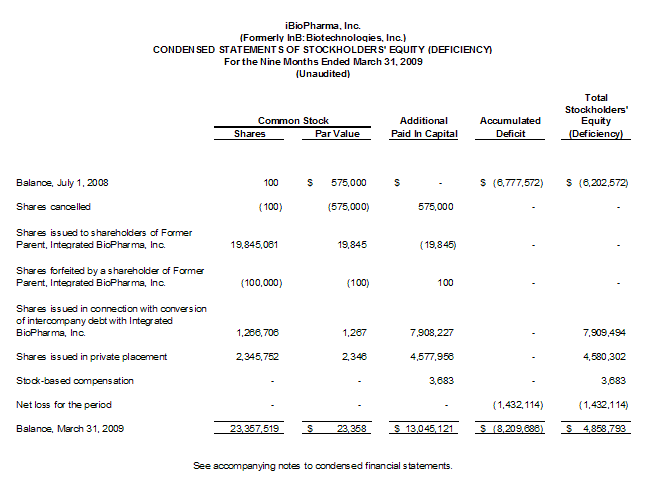
Note 7. Equity Transactions
In November 2007, the Company entered into a Separation and Distribution Agreement (the “Distribution”) with its Former Parent, whereby, the Former Parent agreed to distribute, pro rata, to the holders of its common stock, all of the shares it owned of the Company’s common
stock. The completion of the Distribution was subject to various customary closing conditions, including the declaration by the U.S. Securities and Exchange Commission of the effectiveness of the registration under the Securities Exchange Act of 1934 of the Company’s common stock. The Distribution was completed on August 18, 2008 and each shareholder of our Former Parent received one share of the Company for each share the shareholder owned as of August 12, 2008, the Record Date.
The Distribution should qualify as a tax-free reorganization under Section 355 of the Internal Revenue Code of 1986, as amended. The Separation and Distribution Agreement prohibits the Company from issuing more than 19,845,061 of additional shares of its common stock (representing the number of shares issued in connection with the Distribution) for the two years immediately following the effective date of the Distribution.
Additionally, on August 19, 2008, our Former Parent entered into a Conversion Agreement, whereby Integrated BioPharma caused approximately $5.2 million of the intercompany debt to be contributed to additional paid in capital and used $2.7 million of the intercompany debt to purchase approximately 1.3 million shares of the Company, representing 6% of the then
Page 15
outstanding shares of the Company. Subsequent to the Company’s private placement as discussed below, Integrated BioPharma owns 5.4% of the Company.
Also, on August 19, 2008, the Company closed on its $5.0 million capital raise in connection with its private placement of approximately ten percent (10%) of the Company, such funds were released to the Company from the escrow and issued approximately 2.3 million shares of the Company’s par value $0.001 common stock, at an estimated purchase price of approximately $2.13 per share. The Company’s net proceeds from its private placement were approximately $4.6 million after
payment of certain expenses related to the capital raise.
The Company also issued to the private placement investors, warrants to purchase a number of shares of common stock equal to 50% of the number of shares purchased by such private placement investor, with an exercise price equal to 150% of the purchase price of the Company’s common stock subject to adjustments therein and warrants to purchase a number of shares of common stock equal to 50% of the number of shares purchased by such private placement investor, with an exercise price
equal to 200% of the purchase price of the Company’s common stock subject to adjustments therein and exercisable over the next five-year period.
On February 13, 2009, the Company granted options to members of management and the Board of Directors to purchase 1,080,000 shares of common stock at an exercise price of $0.20 for a period of 10 years. The Company estimated the fair value of these options as of the date of the grant to be $134,820 utilizing the Black-Scholes model and will record stock based compensation expense ratably over the vesting periods ranging from
three to five years. The fair value of these options was estimated at the date of grant using the following assumptions: Dividend yield of 0%; Risk-free interest rate of 1.4% to 1.9%; Volatility of 80%; and Expected life of three to five years. For the three and nine month periods ended March 31, 2009, the Company recorded stock compensation expense of $3,683 for these options.
Note 8. Subsequent Event
Subsequent to March 31, 2009, we entered into an agreement with an effective date of April 1, 2009 wherein we granted to IHT (a wholly owned subsidiary of our Former Parent) an exclusive license to the Company's patented process in consideration for a royalty of five percent (5%) of net sales and the obligation of IHT to maintain in force and good
standing the Company's patent and related intellectual property.
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANICAL CONDITION AND RESULTS OF OPERATION
Certain statements set forth under this caption constitute “forward-looking statements.” See “Disclosure Regarding Forward-Looking Statements” on page 1 of this Report for additional factors relating to such statements. The following discussion should also be read in conjunction with the Condensed Consolidated Financial Statements of the Company and Notes thereto included elsewhere herein and the Company’s Annual Report on Form 10-K.
Overview
iBioPharma, Inc. (formerly, InB:Biotechnologies, Inc.) (the “Company”) is a biopharmaceutical company focused on using and promoting the use of its proprietary plant-based technology platform (the “Platform”) by which targeted proteins can be produced in plants for the development and manufacture of novel vaccines and therapeutics for humans and certain veterinary applications. References in this Quarterly Report, on Form 10-Q, to “we,” “us”, “our company” or “InB:Biotechnologies”, refer to iBioPharma, Inc.. The Platform was invented and
Page 16
developed by Fraunhofer USA Center for Molecular Biotechnology (“FhCMB”), a not-for-profit translational research institution. In January 2004, we acquired from FhCMB the Platform and FhCMB’s commitment for maintenance and support necessary to further protect the
intellectual property comprising the Platform, including filing and prosecuting patent applications, providing scientific support for patent counsel’s activities on behalf of the Company and otherwise to maintain in force and good standing the Company’s intellectual property rights.
Our business model contemplates that we will license the Platform to, or enter into joint ventures or other collaborative arrangements (collectively, “Licenses”) with, other parties (“Licensees”) who wish to use the Platform for the development and/or production of their own product candidates. In order to attract appropriate Licensees and increase the value of the Company’s share of such collaborative arrangements, the Company engaged FhCMB in October
2004, to perform research and development activities to apply the Platform to create a product candidate. The Company selected plant-based flu vaccine for human use as the product candidate to exemplify the value of the Platform particularly for products that require rapid, highly-scalable and economic production. Performance of this first research agreement, which requires us to make payments to FhCMB against the achievement of stated research milestones, has progressed through
preclinical challenge studies in the ferret model.
In addition, in 2006, the Company engaged FhCMB to create a prototype production module for products made through the use of the Platform. The purpose for this engagement was to demonstrate the ease and economy with which Platform-based products could be manufactured, again in order to attract Licensees and increase the value of the Company’s share of collaborative arrangements. The prototype design, which encompasses the entire production process from the seeding through
pre-infiltration plant growth, infiltration with agrobacteria harvesting of plant tissue and purification of target proteins, was completed in May 2008. A pilot plant based on the prototype is being completed in the FhCMB facility in Newark, Delaware. Equipment in the facility is scheduled to be commissioned and the facility validated for cGMP production in the third quarter of calendar 2009. The facility will then be
used for pilot scale production of protein targets for clinical trials of product candidates which use our Platform technology.
In addition to our direct funding of FhCMB’s application of the Platform technology to our human flu vaccine product candidate, we have established arrangements (“Non-Commercial Arrangements”) among the Company, certain government entities (“GEs”), a non-governmental organization (“NGO”) and FhCMB, pursuant to which
the Company grants non-commercial rights to use its Platform for the development and production by FhCMB of product candidates selected by the GEs and NGO, in consideration for grants by the GEs and NGO directly to FhCMB to fund such research and development.
Through the Company/FhCMB contracts and the Non-Commercial Arrangements (collectively, the “Business Structure”), the Company retains ownership of the intellectual property and exclusive commercial rights in the fields of human health and veterinary influenza applications of the intellectual property; but licenses or otherwise grants use rights (i) to GEs and NGO entities for not-for-profit applications of the intellectual property for the development or application of which
they granted funding, and (ii) to FhCMB for research purposes and
Page 17
applications in other fields. This Business Structure is enabling us to obtain commercial rights to various applications of our Platform technology funded by GEs and NGOs. It also helps us demonstrate the validity and apparent value of the Platform to parties to whom we will offer licenses
or collaborative opportunities. Our use of FhCMB to perform research and development work allows us to develop our product candidates, and thereby promote the value of our Platform for licensing and collaboration purposes, without bearing the full risk and expense of establishing and maintaining our own research and development staff and facilities.
Using this Business Structure, we have applied our Platform technology to create a pipeline of proprietary product candidates which we can offer to Licensees, including vaccine and therapeutic candidates against seasonal and pandemic influenza, human papilloma virus (HPV), and other pathogens of public health significance. All of our product candidates are in the preclinical development stage. We sometimes refer to the Platform technology as “iBioLaunch™ technology” or
the “iBioLaunch™ platform,” and we refer to the category of this technology as “plant-based technology” or as a “plant-based platform.”
In January of 2009, the Company and FhCMB agreed to suspend further preparation for clinical trials of a seasonal flu vaccine candidate and instead to focus on clinical trials of a pandemic flu vaccine candidate of interest also to the Bill & Melinda Gates Foundation, which granted FhCMB $8.7 million to fund clinical trials of the pandemic flu candidate based upon the Company’s Platform.
Historically, we have also used plants as sources of high quality nutritional supplements. The Company has a patented process for hydroponic growth of edible plants that causes them to accumulate high levels of important nutritional minerals such as chromium, selenium, iron and zinc. We entered into an agreement with an effective date of April 1, 2009 wherein we granted to IHT Health Products, Inc. (a wholly owned subsidiary of our Former Parent) ("IHT") an exclusive license
to the Company's patented process in consideration for a royalty of five percent (5%) of net sales and the obligation of IHT to maintain in force and good standing the Company’s patent and related intellectual property. At the same time, rights under the Mannatech agreement and other customer agreements have been beneficially transferred to IHT; until formal transfer of the agreements, the Company will act
as IHT’s agent thereunder.
Effect of Spin-off from Integrated BioPharma, Inc.
After the distribution, which occurred on August 18, 2008, the contribution of additional capital from Integrated BioPharma, our Former Parent, and the $5.0 million private placement, Integrated BioPharma owns approximately 5.4% of our common stock, and ceased to control iBioPharma.
Critical Accounting Policies and Estimates
There have been no changes to our critical accounting policies in the nine months ended March 31, 2009. Critical accounting policies and the significant estimates made in accordance with them are regularly discussed with our Audit Committee. Those policies are discussed under
Page 18
“Critical Accounting Policies” in our “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 7 of our Annual Report on Form 10-K for the year ended June 30, 2008.
Results of Operations
Three months ended March 31, 2009 compared to the three months ended March 31, 2008
Net Sales.
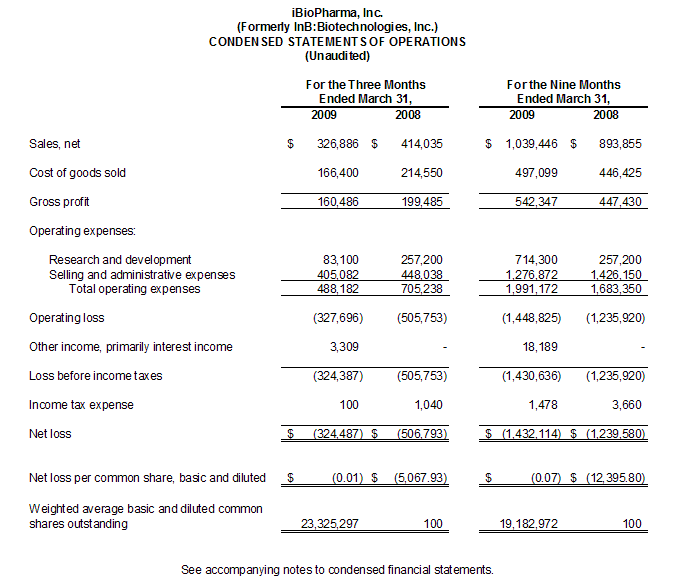
Net sales for the three months ended March 31, 2009 and 2008 were $326,900 and $414,000, respectively, a decrease of $87,100 or 21%. Sales under our
supply agreement with Mannatech represented 58% of our sales in the three month period ended March 31, 2009 compared to 93% of our net sales in the three month period ended March 31, 2008.
For the three months ended March 31, 2009, substantially all of net sales were derived from two customers. Natural Alternatives International (58%), became our customers under our supply agreement with Mannatech at the direction of Mannatech for the purpose of supplying certain raw
materials in the manufacturing process of Mannatech’s nutraceutical product lines. The other customer, FhCMB represented 44% of net sales for the period ended March 31, 2009 and relates to our subcontract agreement with FhCMB under their DARPA (Defense Advanced Research Agency)
grant. For the three months ended March 31, 2008, substantially all of our net sales were derived from two customers: L. Perrigo Company (40%) and Natural Alternatives International (53%) both in connection with our supply agreement with Mannatech. The loss of
any of these customers would have an adverse affect on our net sales.
Cost of sales.
Cost of sales decreased to $166,400 for the three months ended March 31, 2009, as compared to $214,550 for the three months ended March 31, 2008. Cost of sales represented 51% and 52% of sales during the three month periods ended March 31, 2009 and 2008, respectively, and are attributable to our manufacturing oversight contract with IHT for sales under the supply agreement with Mannatech. Our subcontract agreement with FhCMB has no cost of sales.
Research and Development Costs.
Our research and development costs were $83,100 in the three months ended March 31, 2009 compared to $257,200 in the three months ended March 31, 2008. Research and development costs consist primarily of payments made or owed to FhCMB in reaching milestones under our research agreements with them. The absence of such costs during the three months ended March 31, 2009 reflect suspension of work under that research agreement in favor of pandemic flu vaccine candidate trials which are financed by a grant from the Bill and Melinda Gates Foundation to FhCMB and utilize the Company's technology licensed to FhCMB.
Page 19
Selling and Administrative Expenses.
Selling and administrative expenses were $405,100 for the three months ended March 31, 2009, an increase of $42,900 as compared with $448,000 for the three months ended March 31, 2008. A tabular presentation of the changes in selling and administrative expenses is as follows:
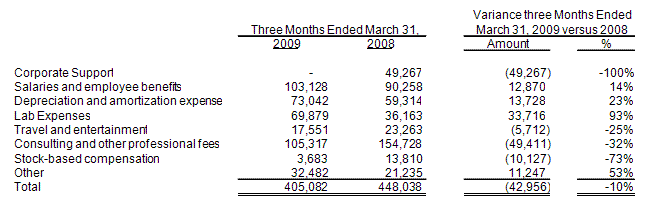
Corporate support charges from Integrated BioPharma were zero in the three months ended March 31, 2009 and were approximately $49,300 for the three months ended March 31, 2008. Integrated BioPharma ceased charging us the corporate support charges subsequent to the spin-off from Integrated BioPharma on August 18, 2008.
Corporate support charges for the three months ended March 31, 2008 consisted of the following:

The salary allocation was an allocation of the Integrated BioPharma’s salaries and related employee costs for persons in the executive management team that devoted a portion of their time to iBioPharma’s business and an allocation of the accounting and support staff of Integrated BioPharma whom also devoted a portion of their time to our record keeping and administrative matters. The overhead allocation was an allocation of Integrated BioPharma’s allocable overhead accounts including office expenses, telephone, professional fees, consulting fees, finance charges and travel and entertainment expenses and were allocated to each of Integrated BioPharma’s subsidiaries’ based on the estimated percentage of time devoted to each company, including Integrated BioPharma, and actual expenses of Integrated BioPharma on a trailing six month period.
Salaries and employee benefits increased to approximately $103,100 in the three months ended March 31, 2009 from approximately $90,300 in the three months ended March 31, 2008, an increase of approximately $12,800. The increase is primarily attributable to the increased salary
Page 20
and benefits costs for our Chief Executive Officer and his assistant, whose costs were previously charged through the Corporate Support charges.
Depreciation and amortization expense increased to approximately $73,000 in the three months ended March 31, 2009 from approximately $59,300 in the three months ended March 31, 2008, or approximately $13,700 or 23%. The primary increase is attributable to additional intangible assets of approximately $522,200 period over period in patents.
In the three months ended March 31, 2009, lab expense increased by $33,700 to $69,900 from $36,200 in the comparable period a year ago. This change was attributable to increased lab salaries of approximately $60,800 related to the hiring of additional employees to work on lab projects, primarily related to our grant income under our FhCMB agreement, offset by a decrease in other lab expenses of $27,100.
Travel and entertainment expense decreased to approximately $5,700 in the three months ended March 31, 2009 from approximately $23,300 in the three months ended March 31, 2008, or approximately $17,600 or 65%. This change was attributable to the elevated level of travel in the prior period related to fundraising activities.
Consulting and other professional fees decreased by approximately $49,400 or 32% in the three months ended March 31, 2009 to approximately $105,300 compared to approximately $154,700 in the three months ended March 31, 2008. Consulting and other professional fees consist of legal, outside accounting services, director’s fees, scientific advisory board (“SAB”) expenses (both travel and consulting fees) and consulting fees paid to outside consultants and our own Chief Scientific Officer. The decrease of $49,400 in consulting and other professional fees, , from the three months ended March 31, 2009 compared to March 31, 2008 was primarily the result of decreases in accounting fees of $12,900, legal fees of $52,000 and directors' fees of $4,700 offset by increases in transitional fees of $25,000.
Pursuant to SFAS No. 123(R), adopted as of July 1, 2005, we recognized approximately $3,700 and $14,100 in compensation expense for employee stock options in the three months ended March 31,
2009 and 2008, respectively. The expense in the three months ended March 31, 2008 was a direct allocation from our Former Parent for our employees and directors who received compensation in the form of stock options providing for the purchase of our Former Parent’s stock upon vesting of their awards.
Other expense increased to approximately $32,500 in the three months ended March 31, 2009
from approximately $21,200 in the three months ended March 31, 2008, approximately $11,300 or 53%. As a percentage of total selling and administrative expenses, other expenses were 7% and 5% in the three months ended March 31, 2009 and 2008,
respectively.
Income tax (benefit).
In the three months ended March 31, 2009, the Company had net income tax expense of approximately $100 and $1,000 in the three months ended March 31, 2009 and 2008,
Page 21
respectively. Our ability to recognize an income tax benefit was dependent on the consolidated federal taxable income (loss) of Integrated BioPharma’s controlled group for federal income tax purposes. In the three months ended March 31, 2008, the controlled group of Integrated BioPharma had a taxable loss and therefore did not utilize any of the losses generated by us and as a stand-alone taxable entity. For the three months ended March 31, 2009, we reserved 100% of our resulting deferred tax asset generated from the net operating loss as it is more likely than not that, in the near term, that we will not generate sufficient taxable income to offset our taxable losses in the periods presented. Our deferred tax asset relating to our federal and state net operating losses are fully reserved in a valuation allowance account since it is more likely than not that we will not have sufficient taxable income, in the near future, to offset any future taxable income. The income tax expense recognized in our statement of operations represents minimum state income taxes due in the states we are required to file income tax returns.
Nine months ended March 31, 2009 compared to the nine months ended March 31, 2008
Net Sales.
Net sales for the nine months ended March 31, 2009 and 2008 were $1,039,400 and $893,900, respectively, an increase of $150,400 or 17%. Sales under our supply agreement with Mannatech represent 56% of our sales in the nine month period ended March 31, 2009 compared to 95% of our net sales in the nine month period ended March 31, 2008.
For the nine months ended March 31, 2009, substantially all of net sales were derived from three customers. Two of these customers, L. Perrigo Company (16%) (formerly, JB Laboratories, Inc.) and Natural Alternatives International (40%), became our customers under our supply agreement with Mannatech at the direction of Mannatech for the purpose of supplying certain raw materials in the manufacturing process of Mannatech’s nutraceutical product lines. The remaining customer, FhCMB represents 43% of net sales for the period ended March 31, 2009 and relates to our subcontract agreement with FhCMB under their DARPA (Defense Advanced Research Agency) grant. For the nine months ended March 31, 2008, substantially all of our net sales were derived from two customers: L. Perrigo Company (45%) and Natural Alternatives International (50%) all in connection with our supply agreement with Mannatech. The loss of any of these customers would have an adverse affect on our net sales.
Cost of sales.
Cost of sales increased to $497,100 for the nine months ended March 31, 2009, as compared to $446,400 for the nine months ended March 31, 2008. Cost of sales represented 48% and 49% of sales during the nine month periods ended March 31, 2009 and 2008, respectively, and are attributable to our manufacturing oversight contract with IHT for sales under the supply agreement with Mannatech. Our subcontract agreement with FhCMB has no cost of sales.
Page 22
Research and Development Costs.
Our research and development costs were $714,300 in the nine months ended March 31, 2009 compared to $257,200 in the nine months ended March 31, 2008. Research and development costs consist primarily of payments made or owed to FhCMB in reaching milestones under our research agreements with them. The increase of $457,100 was the result in a $250,000 increase of payments made to FhCMB under our research agreements with them and increased salary and benefits costs of $207,000 in the nine months ended March 31, 2009 compared to the nine months ended March 31, 2008.
Selling and Administrative Expenses.
Selling and administrative expenses were $1,276,900 for the nine months ended March 31, 2009, a decrease of $149,300 as compared with $1,426,200 for the nine months ended March 31, 2008. A tabular presentation of the changes in selling and administrative expenses is as follows:
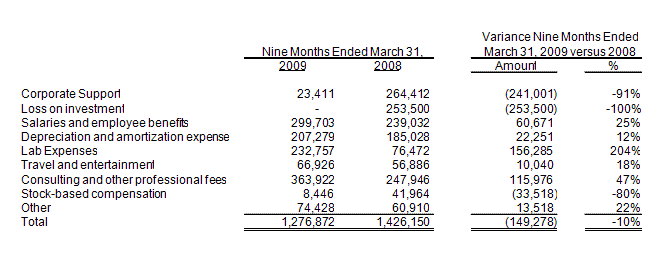
Corporate support charges from Integrated BioPharma were $23,400 in the nine months ended March 31, 2009 and were approximately $264,400 for the nine months ended March 31, 2008. Integrated BioPharma ceased charging us the corporate support charges subsequent to the spin-off from Integrated BioPharma on August 18, 2008.
Corporate support charges consisted of the following:

The salary allocation was an allocation of the Integrated BioPharma’s salaries and related employee costs for persons in the executive management team that devoted a portion of their time to iBioPharma’s business and an allocation of the accounting and support staff of Integrated
Page 23
BioPharma whom also devoted a portion of their time to our record keeping and administrative matters. The overhead allocation was an allocation of Integrated BioPharma’s allocable overhead accounts including office expenses, telephone, professional fees, consulting fees, finance charges and travel and entertainment expenses and were allocated to each of Integrated BioPharma’s subsidiaries’ based on the estimated percentage of time devoted to each company, including Integrated BioPharma, and actual expenses of Integrated BioPharma on a trailing six month period.
In December 2006, the Company made in an investment in a private biotech company that was in its initial stages of filing to become a public company. In the nine month period ended March 31,
2008, the Company, based in part on information from public filings of the biotech company, charged off its entire investment, $253,500, in this biotech company.
Salaries and employee benefits increased to approximately $299,700 in the nine months ended March 31, 2009 from approximately $239,000 in the nine months ended March 31, 2008, an increase of approximately $60,700. The increase is primarily attributable to the increased salary cost for our President who was employed for the full nine months in the period ended March 31, 2009 versus six in the year ago period.
Depreciation and amortization expense increased to approximately $207,300 in the nine months ended March 31, 2009 from approximately $185,000 in the nine months ended March 31, 2008, or approximately $22,300 or 12%. The primary increase is attributable to additional intangible assets of approximately $522,200 period over period in patents.
In the nine months ended March 31, 2009, lab expense increased by $156,300 to $232,800 from $76,500 in the comparable period a year ago, substantially all the increase relates to salaries of employees charged to lab expense. The increase in lab salaries was a result of hiring additional employees to work on lab projects, primarily related to our grant income under our FhCMB agreement, in the nine months ended March 31, 2009 compared to the prior year.
Travel and entertainment expenses increased by $10,000 to $66,900 in the nine months ended March 31, 2009 compared to $56,900 in the nine months ended March 31, 2008. Such costs relate to travel by our president who resides in California, and our Chief Scientific Officer, who resides in London, and members of our SAB, who travel in connection with their visits to our offices in Delaware and to attend various meetings in New York and Florida.
Consulting and other professional fees increased by approximately $116,000 or 47% in the nine months ended March 31, 2009 to approximately $363,900 compared to approximately $247,900 in the nine months ended March 31, 2008. Consulting and other professional fees consist of legal, outside accounting services, director’s fees, scientific advisory board (“SAB”) expenses (both travel and consulting fees) and consulting fees paid to outside consultants and our own Chief Scientific Officer. This increase was primarily the result of increases in legal fees of $47,200, audit fees of $57,800, and transitional fees of $61,900 offset by decreases in public relations fees of $27,600 and scientific advisory board fees of $26,200. Legal fees in the nine months ended March 31, 2008 were unusually low since we had negotiated discounts with our
Page 24
legal firms for legal fees incurred in that period and prior periods estimated at approximately $224,500 and recorded the estimated recovery of those legal fees in that period
Pursuant to SFAS No. 123(R), adopted as of July 1, 2005, we recognized approximately $8,400 in compensation expense for employee stock options in the nine months ended March 31, 2009 and $42,000 in the nine months ended March 31, 2008. The expense in the nine months ended March 31, 2008 was a direct allocation from our Former Parent for our employees and directors who received compensation in the form of stock options providing for the purchase of our Former Parent’s stock upon vesting of their awards.
Other expense increased to approximately $74,400 in the nine months ended March 31, 2009 from
approximately $60,900 in the nine months ended March 31, 2008, approximately $13,500 or 22%. As a percentage of total selling and administrative expenses, other expenses were 5% and 4% in the nine months ended March 31, 2009 and 2008, respectively.
Income tax (benefit).
In the nine months ended March 31, 2009, the Company had net income tax expense of approximately $1,500 compared to $3,700 in the nine months ended March 31, 2008. Our ability to recognize an income tax benefit was dependent on the consolidated federal taxable income (loss) of Integrated BioPharma’s controlled group for federal income tax purposes. In the nine months ended March 31, 2008, the controlled group of Integrated BioPharma had a taxable loss and therefore did not utilize any of the losses generated by us and as a stand-alone taxable entity. For the nine months ended March 31, 2009, we reserved 100% of our resulting deferred tax asset generated from the net operating loss as it is more likely than not that, in the near term, that we will not generate sufficient taxable income to offset our taxable losses in the periods presented. Our deferred tax asset relating to our federal and state net operating losses are fully reserved in a valuation allowance account since it is more likely than not that we will not have sufficient taxable income, in the near future, to offset any future taxable income. The income tax expense recognized in our statement of operations represents minimum state income taxes due in the states we are required to file income tax returns.
Seasonality
We do not believe that our operations are impacted by seasonality.
Page 25
Liquidity and Capital Resources
The following table sets forth, for the periods indicated, the Company’s net cash flows used in operating, investing and financing activities:
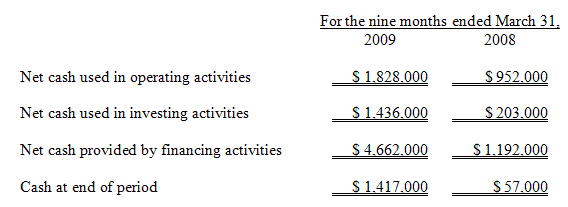
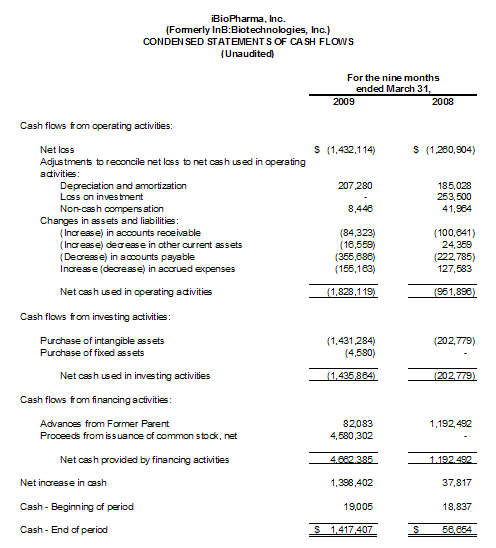
At March 31, 2009, we had working capital of $1,299,000, an increase from our negative working capital of $1,761,000 as of June 30, 2008. Our cash position increased significantly from June 30, 2008 as a result of the $4,580,000 of net proceeds we received from our private placement of common stock in August 2008.
In the nine months ended March 31, 2009, we used $1,828,000 of cash in our operating activities compared to $952,000 of cash in operations in the nine months ended March 31, 2008, an increase of approximately $876,000. This increase in use of cash is composed primarily of an increase in our operating loss of $436,000 (our Net Loss adjusted for depreciation and amortization, loss on investment, and non-cash compensation) and reductions in accounts payable of $133,000 and accrued expenses of $283,000.
The decreases in accounts payable, accrued expenses, and due to Fraunhofer is primarily attributable to use of proceeds from our $5.0 million private placement in August 2008 to pay down liabilities which had been building through March 31, 2008.
The increase in cash used in investing activities of approximately $1,233,000 in the nine months ended March 31, 2009 compared to the nine months ended March 31, 2008 is primarily due to additions to the patent portfolio.
The increase in cash provided from financing activities of approximately $3,470,000 in the nine months ended March 31, 2009 compared to the nine months ended March 31 2008 is a result of the net proceeds of $4,580,000 from our completion of the $5.0 million private placement of capital completed in August 2008 offset by a net decrease in advances from our Former Parent of $1,110,000.
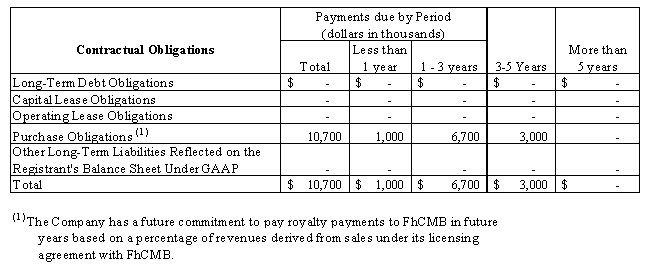
The following table sets forth the Company’s future commitments as of March 31, 2009 (Purchase Obligations represents our expected payments to FhCMB under our amended technology transfer and research agreements):
Page 26
Our plans to expand our business and to continue to improve our product candidates to strengthen our ability to obtain licensees for our proprietary technology may require funds in excess of our cash flow and may require us to seek financing from third parties. In the past, Integrated BioPharma has provided capital for our general corporate purposes, and we used cash provided by Integrated BioPharma to fund our operations. Since the distribution, Integrated BioPharma has not and will not provide funds to finance our operations. Without the opportunity to obtain financing from Integrated BioPharma, we will in the future need to obtain additional financing from banks, or through public offerings or private placements of debt or equity securities, strategic relationships or other arrangements. The terms, interest rates, costs and fees of new credit facilities may not be as favorable as those historically enjoyed with Integrated BioPharma. For example, Integrated BioPharma did not charge us with any fees or costs for the intercompany borrowing, nor were there any covenants regarding financial ratios or prohibition on certain transactions in the loan arrangement with Integrated BioPharma. Our inability to obtain financing on favorable terms could restrict our operations and increase our losses.
In August 2008, we closed on our $5.0 million private placement, which funds were released from an escrow account subsequent to the spin-off. This additional capital is expected to cover our anticipated costs through the first quarter of calendar year 2010. If we are unsuccessful in raising additional capital or other alternative financing by then we might have to postpone or abandon our efforts to commercialize the intellectual property and suspend operations.
Capital Expenditures
The Company’s capital expenditures, other than intellectual property, during the nine months ended March 31, 2009 and 2008 were not material.
Page 27
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Recently Announced Accounting Pronouncements
Please refer to Note 2 in our financial statements, which can be found at page 6, herein.
The Company does not believe that inflation has significantly affected its results of operations.
Item 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
In the normal course of business, the Company may be a party to financial instruments that are subject to market risks arising from changes in interest rates and foreign currency rates. We currently do not use derivative financial instruments to address treasury risk management issues in connection with changes in interest rates and foreign currency rates.
Item 4T. CONTROLS AND PROCEDURES
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, or the Exchange Act, is recorded, processed, summarized and reported within the time
periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
As required by Rule 13a-15(b) under the Exchange Act, we carried out an evaluation, under the supervision and with the participation of management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing, our principal executive officer and principal financial officer concluded that, as of the end of the period covered by this report, our disclosure controls and procedures were effective at the reasonable assurance level to ensure that information required to be disclosed by us in reports that we file under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. There have been no changes in our internal control over financial reporting during the fiscal quarter ended March 31, 2009 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Page 28
PART II – OTHER INFORMATION
We are not currently a party to any material legal proceedings.
The risks described in Item 1A, Risk Factors, in our Annual Report on Form 10-K for the year ended June 30, 2008, could materially and adversely affect our business, financial condition and results of operations. The risk factors discussed in that Form 10-K do not identify all risks that we
face because our business operations could also be affected by additional factors that are not presently known to us or that we currently consider to be immaterial to our operations.
Current economic conditions may cause a decline in business and consumer spending which could adversely affect our business and financial performance.
Our operating results are impacted by the health of the North American economies. Our business and financial performance, including collection of our accounts receivable, recoverability of assets including investments, may be adversely affected by current and future economic conditions, such
as a reduction in the availability of credit, financial market volatility, recession, etc.
Additionally, we may experience difficulties in scaling our operations to react to economic pressures in the U.S.
Item 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
Item 3. DEFAULTS UPON SENIOR SECURITIES
None.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
None.
None.
Page 29
(a) Exhibits
Exhibit
Number
|
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 by Chief Executive Officer. |
|
|
31.2 |
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 by Chief Financial Officer |
|
32.1 |
Certification of periodic financial report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 by Chief Executive Officer. |
|
32.2 |
Certification of periodic financial report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 by Chief Financial Officer. |
Page 30
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Company has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
iBioPharma, Inc. |
|||
|
Date: May 15, 2009 |
By: /s/ Robert B. Kay |
||
|
Robert B. Kay, |
|||
|
Chief Executive Officer |
|||
|
Date: May 15, 2009 |
By: /s/ Dina L. Masi |
||
|
Dina L. Masi, |
|||
|
Interim Chief Financial Officer |
Page 31
Exhibit 31.1
Certification of Chief Executive Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Robert B. Kay certify that:
1. I have reviewed this quarterly report on Form 10-Q of iBioPharma, Inc. for the quarter ended March 31, 2009;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reports (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
|
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
c) |
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
d) |
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting. |
5. The registrant's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
|
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and |
|
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting. |
|
Date: May 15, 2009 |
By: /s/ Robert B. Kay |
||
|
Name: Robert B. Kay, |
|||
|
Title: Chief Executive Officer |
Exhibit 31.2
Certification of Chief Financial Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Dina L. Masi, certify that:
1. I have reviewed this quarterly report on Form 10-Q of iBioPharma, Inc. for the quarter ended March 31, 2009;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reports (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
|
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
c) |
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
d) |
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting. |
5. The registrant's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
|
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and |
|
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting. |
|
Date: May 15, 2009 |
By: /s/ Dina L. Masi |
||
|
Name: Dina L. Masi |
|||
|
Title: Interim Chief Financial Officer |
Exhibit 32.1
CERTIFICATION OF PERIODIC REPORT
As adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Quarterly Report on Form 10-Q for the quarter ended March 31, 2009 of iBioPharma, Inc. (the “Company”) as filed with the Securities and Exchange Commission on the date hereof (the “Report”), Robert B. Kay, the Chief Executive Officer of iBioPharma, Inc. certifies, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350, that to his knowledge:
|
(1) |
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)); and |
|
(2) |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
|
Date: May 15, 2009 |
By: /s/ Robert B. Kay |
||
|
Robert B. Kay, |
|||
|
Chief Executive Officer |
Exhibit 32.2
CERTIFICATION OF PERIODIC REPORT
As adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
In connection with the Quarterly Report on Form 10-Q for the quarter ended March 31, 2009 of iBioPharma, Inc. (the “Company”) as filed with the Securities and Exchange Commission on the date hereof (the “Report”), Dina L. Masi, the Interim Chief Financial Officer of iBioPharma, Inc. certifies, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350, that to her knowledge:
|
(1) |
the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)); and |
|
(2) |
the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
This certification accompanies the Report pursuant to Section 906 of Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by the Sarbanes-Oxley Act of 2002, be deemed filed by the Company for purposes of Section 18 of the Securities Exchange Act
of 1934, as amended.
A signed original of this written statement has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
|
Date: May 15, 2009 |
By: /s/ Dina L. Masi |
||
|
Dina L. Masi |
|||
|
Interim Chief Financial Officer |