Item 7.01. Regulation FD Disclosure.
On October 30, 2025, iBio, Inc. (the “Company”) issued a press release announcing new preclinical data from its obese non-human primate (NHP) study evaluating IBIO-610, potentially a first-in-class Activin E antibody candidate supported by preclinical data. The data will be presented by Cory Schwartz, Ph.D., Director of Research and Early Development of the Company, during an oral session at ObesityWeek 2025, taking place November 4, 2025 through November 7, 2025 in Atlanta.
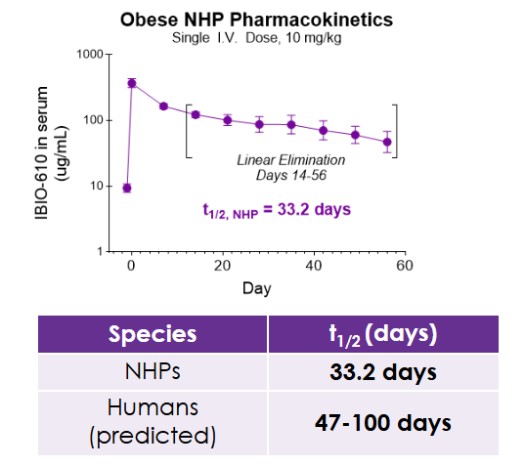
Results from the obese NHP study evaluating IBIO-610 are set forth below:
The pharmacokinetic data, to be presented at ObesityWeek, demonstrates IBIO-610 has an extended half-life in obese NHP of 33.2 days. Based on an allomeric scaling model of half-life extended antibodies, it is predicted IBIO-610 will have a half-life in humans of up to 100 days, reducing the dosing frequency to once every six months, which has the potential to significantly improve patient experience.
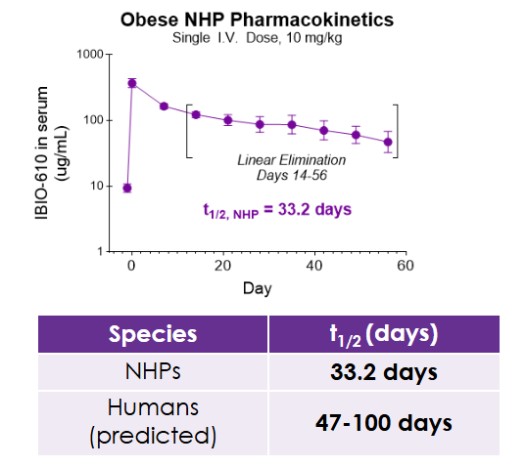
PK of IBIO-610 in NHPs and Allometric Scaling Predicting Human Half-life
The information in this Item 7.01 and in the press release furnished as Exhibit 99.1 to this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended and shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
The press release furnished as Exhibit 99.1 to this Current Report on Form 8-K includes “safe harbor” language pursuant to the Private Securities Litigation Reform Act of 1995, as amended, indicating that certain statements contained therein are “forward-looking” rather than historical.
Item 8.01. Other Events.
On October 30, 2025, the Company issued a press release announcing new preclinical data from its obese NHP study evaluating IBIO-610, potentially a first-in-class Activin E antibody candidate supported by preclinical data. The data will be presented by Cory Schwartz, Ph.D., Director of Research and Early Development of the Company, during an oral session at ObesityWeek 2025, taking place November 4, 2025 through November 7, 2025 in Atlanta.