| Growing Tomorrow’s Biologics CORPORATE PRESENTATION March 2022 Tom Isett, Chairman & CEO © 2022 iBio , Inc. All Rights Reserved. |
| 2 Forward - Looking Statements Certain statements in this presentation constitute "forward - looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended .. Words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," "predict," "forecast," "project," "plan," "intend" or similar expressions, or statements regarding intent, belief, or current expectations, are forward - looking statements .. These forward - looking statements are based upon current estimates .. While the Company believes these forward - looking statements are reasonable, undue reliance should not be placed on any such forward - looking statements, which are based on information available to us on the date of this presentation .. These forward - looking statements are subject to various risks and uncertainties, many of which are difficult to predict that could cause actual results to differ materially from current expectations and assumptions from those set forth or implied by any forward - looking statements .. Important factors that could cause actual results to differ materially from current expectations include, among others, the Company’s ability to obtain regulatory approvals for commercialization of its product candidates, including its COVID - 19 vaccines and IBIO - 101 , or to comply with ongoing regulatory requirements, regulatory limitations relating to its ability to promote or commercialize its product candidates for specific indications, acceptance of its product candidates in the marketplace and the successful development, marketing or sale of products, its ability to maintain its license agreements, the continued maintenance and growth of its patent estate, its ability to establish and maintain collaborations, its ability to obtain or maintain the capital or grants necessary to fund its research and development activities, competition, its ability to retain its key employees or maintain its NYSE American listing, and the other factors discussed in the Company’s most recent Annual Report on Form 10 - K and the Company’s subsequent filings with the SEC, including subsequent periodic reports on Forms 10 - Q and 8 - K .. The information in this presentation is provided only as of today, and we undertake no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law .. This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, nor will there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such state or jurisdiction .. |
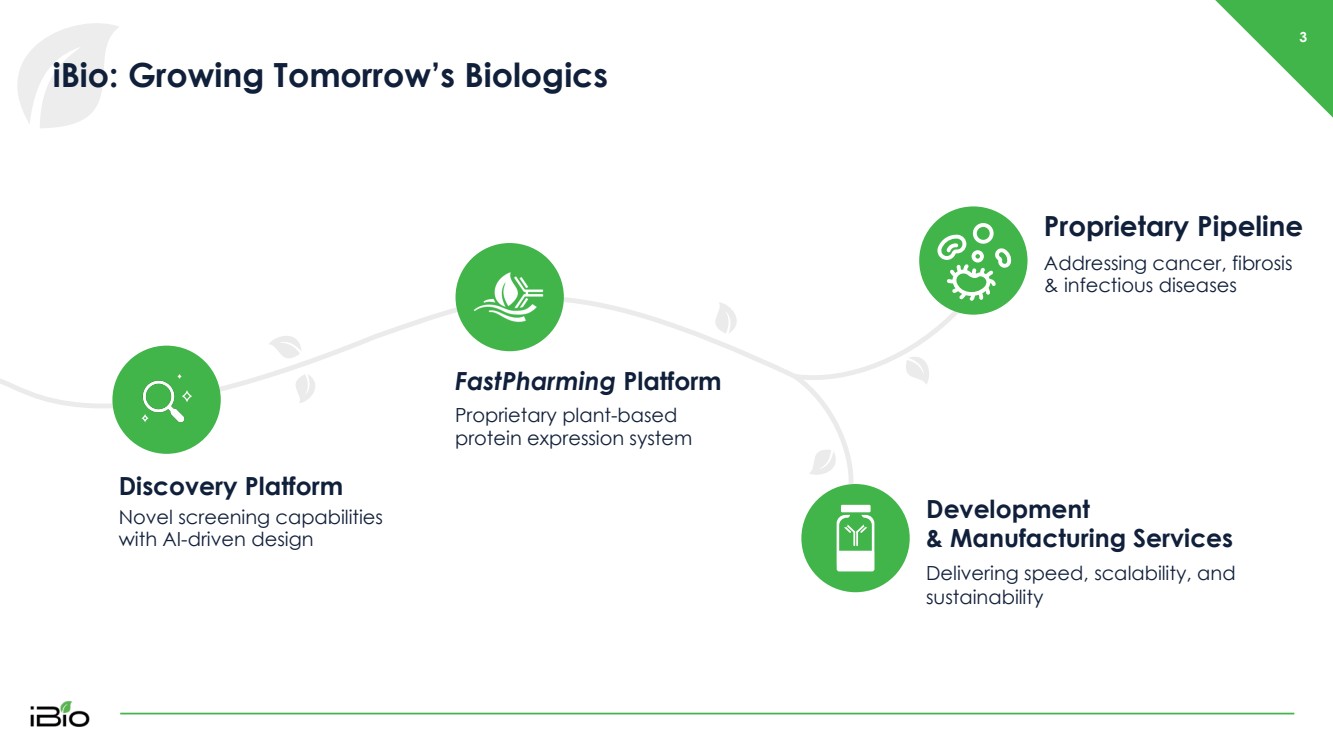
| iBio : Growing Tomorrow’s Biologics Discovery Platform Novel screening capabilities with AI - driven design FastPharming Platform Proprietary plant - based protein expression system Development & Manufacturing Services Delivering speed, scalability, and sustainability Proprietary Pipeline Addressing cancer, fibrosis & infectious diseases 3 |
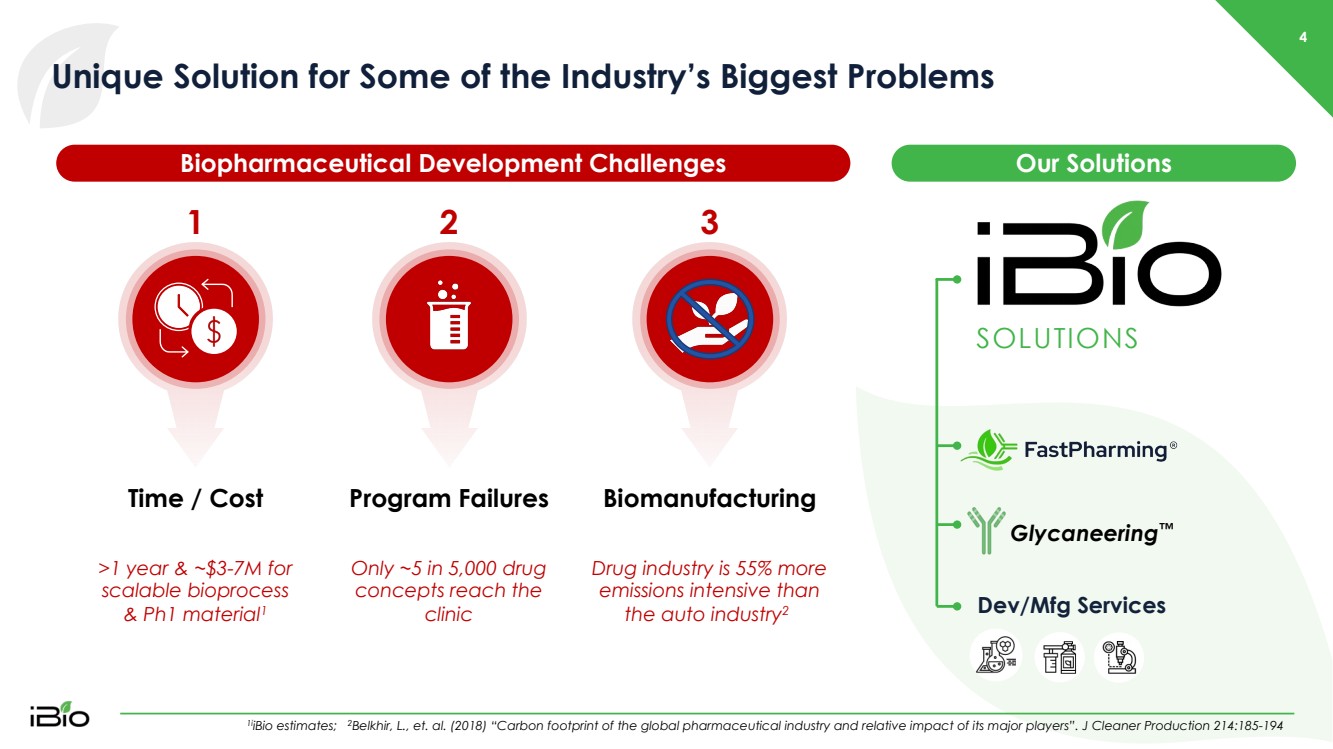
| Only ~5 in 5,000 drug concepts reach the clinic Program Failures 4 Unique Solution for Some of the Industry’s Biggest Problems 1i iBio estimates; 2 Belkhir, L., et. al. (2018) “Carbon footprint of the global pharmaceutical industry and relative impact of its major players” .. J Cleaner Production 214:185 - 194 >1 year & ~$3 - 7M for scalable bioprocess & Ph1 material 1 Time / Cost Biopharmaceutical Development Challenges Drug industry is 55% more emissions intensive than the auto industry 2 Biomanufacturing Glycaneering ™ SOLUTIONS Dev/ Mfg Services Our Solutions 1 2 3 |
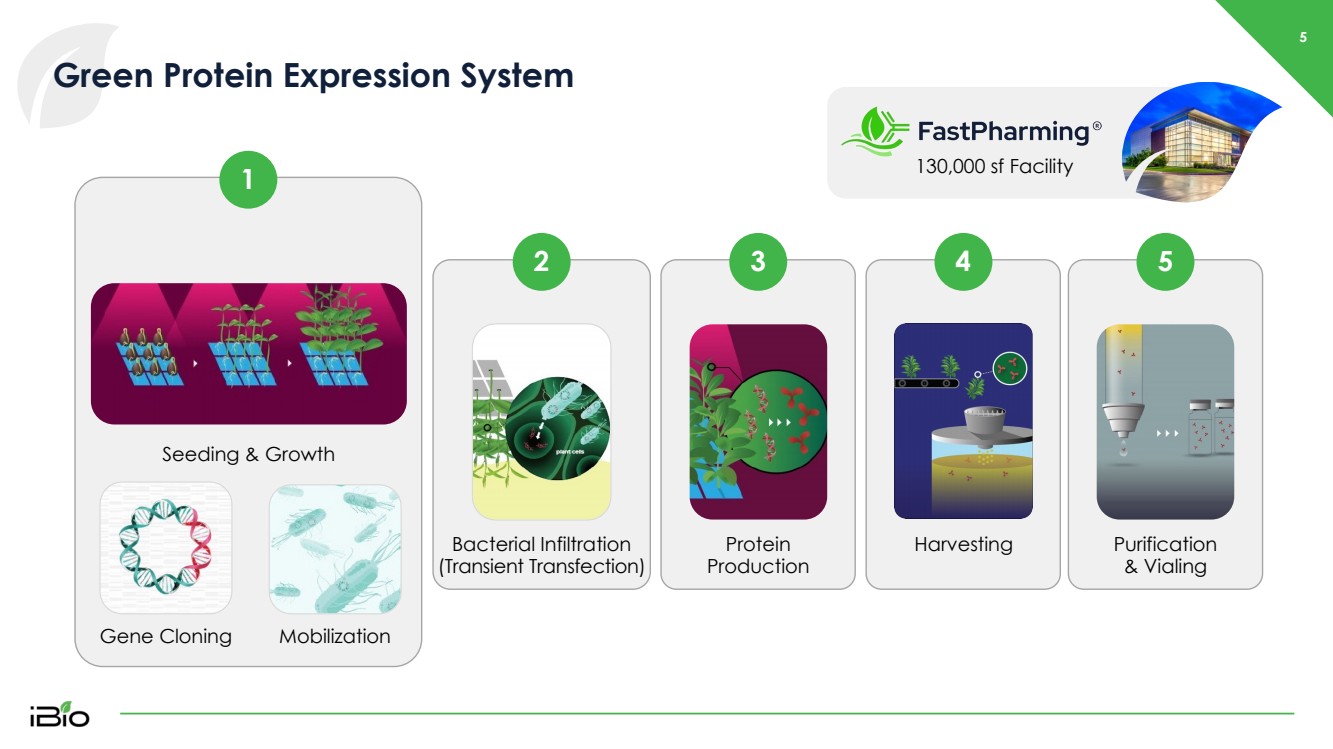
| 5 Green Protein Expression System 130,000 sf Facility 1 Bacterial Infiltration (Transient Transfection) Protein Production Harvesting Purification & Vialing Seeding & Growth Mobilization Gene Cloning 2 3 4 5 |
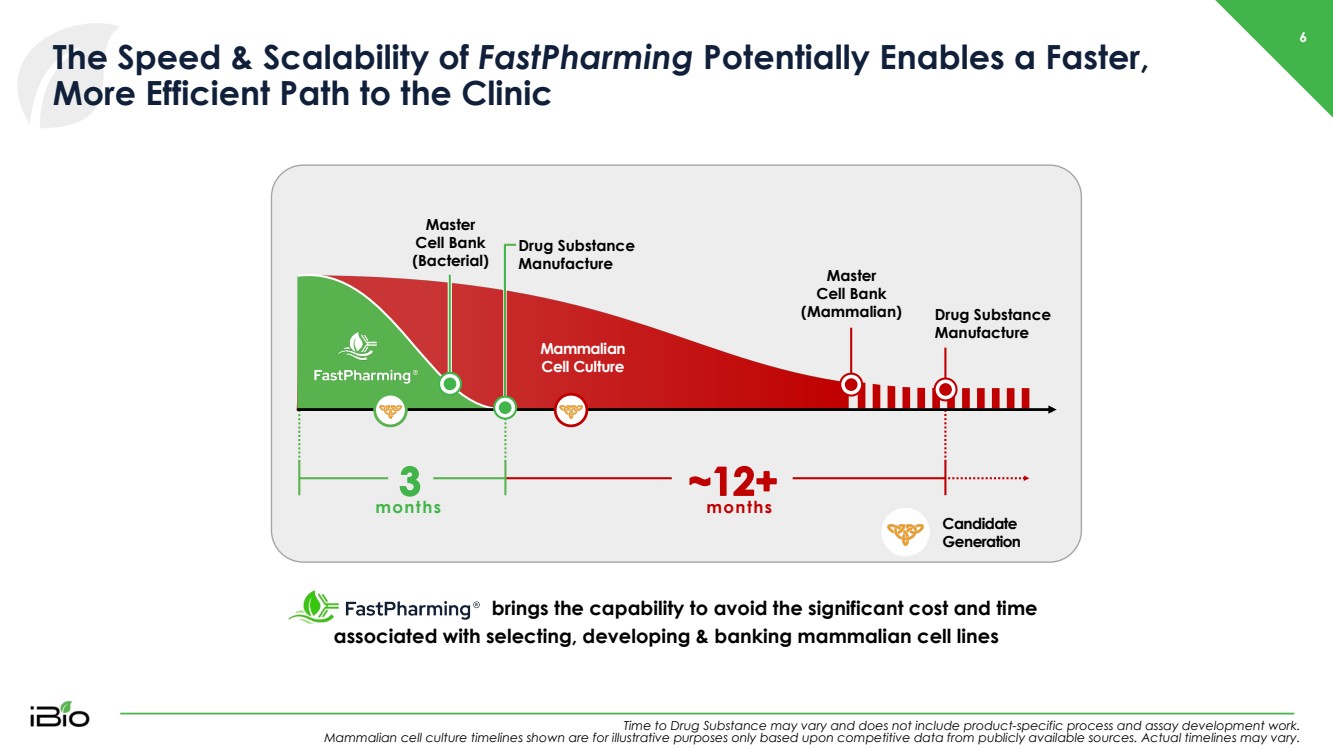
| 6 The Speed & Scalability of FastPharming Potentially Enables a Faster, More Efficient Path to the Clinic Time to Drug Substance may vary and does not include product - specific process and assay development work. Mammalian cell culture timelines shown are for illustrative purposes only based upon competitive data from publicly available so urces. Actual timelines may vary. brings the capability to avoid the significant cost and time associated with selecting, developing & banking mammalian cell lines Master Cell Bank (Mammalian) Drug Substance Manufacture Drug Substance Manufacture Mammalian Cell Culture Master Cell Bank (Bacterial) months months Candidate Generation |
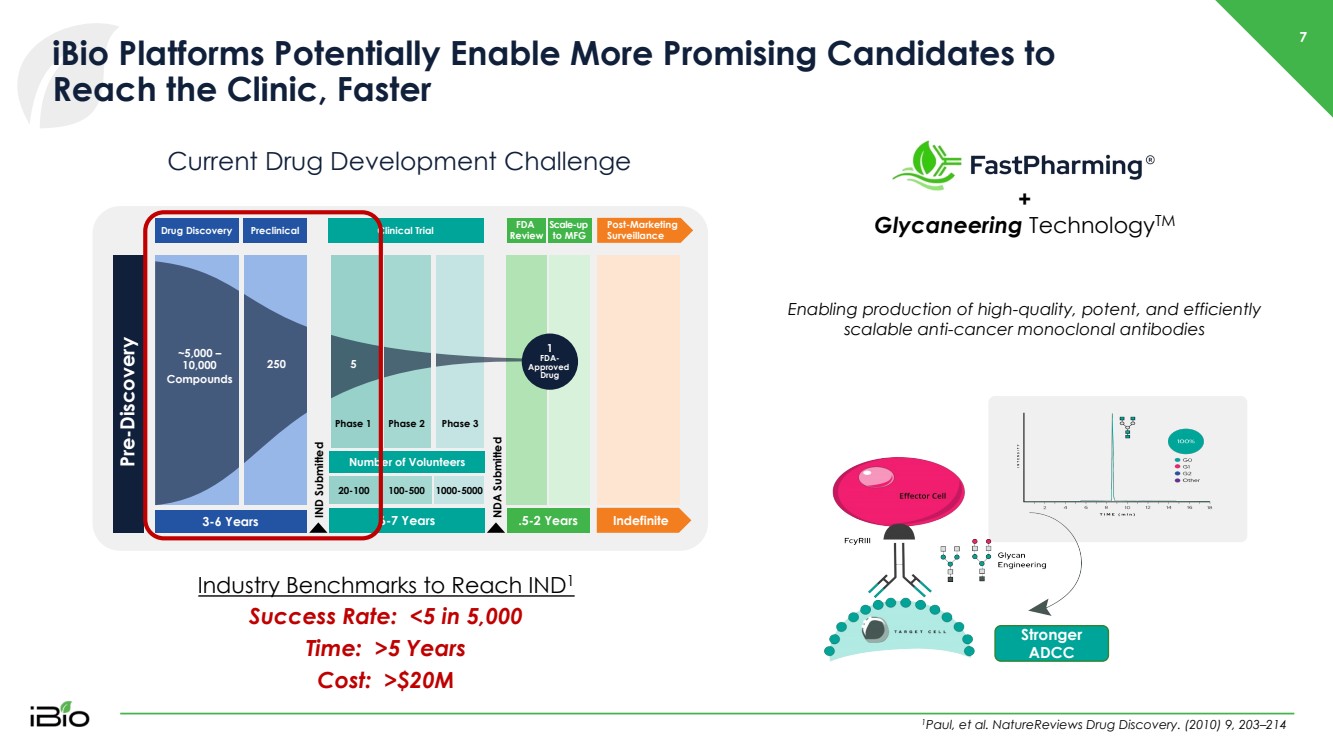
| 7 iBio Platforms Potentially Enable More Promising Candidates to Reach the Clinic, Faster Industry Benchmarks to Reach IND 1 Success Rate: <5 in 5,000 Time: >5 Years Cost: >$20M Current Drug Development Challenge Drug Discovery Preclinical 3 - 6 Years FDA Review Scale - up to MFG Post - Marketing Surveillance NDA Submitted ..5 - 2 Years Indefinite 20 - 100 100 - 500 1000 - 5000 6 - 7 Years Clinical Trial IND Submitted Pre - Discovery Phase 1 Phase 2 Phase 3 Number of Volunteers 1 FDA - Approved Drug ~5,000 – 10,000 Compounds 250 5 1 Paul, et al. NatureReviews Drug Discovery. (2010) 9, 203 – 214 + Glycaneering Technology TM Enabling production of high - quality, potent, and efficiently scalable anti - cancer monoclonal antibodies Stronger ADCC |
| 8 1 17th Annual Report and Summary of Biopharmaceutical Manufacturing Capacity and Production https://www.biopharma.com/TRENDS.pdf .. 2 Seeds, stone wool, and purified water FastPharming : Reducing Single - Use Plastic Disposables in Bioprocessing The FastPharming Expression System uses all - natural raw materials 2 VS >85% of pre - commercial bioprocesses involve single - use plastic disposables 1 |
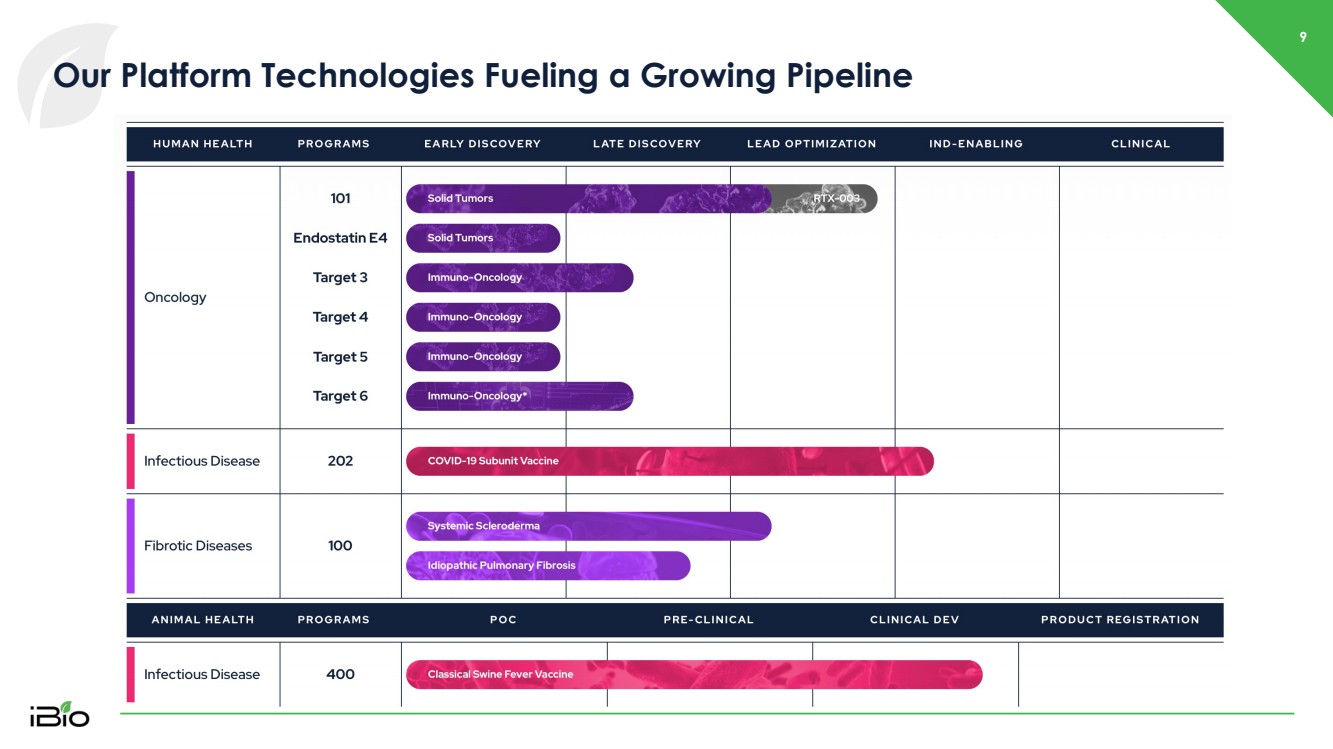
| 9 Our Platform Technologies Fueling a Growing Pipeline |
| Therapeutics Oncology & Fibrosis |
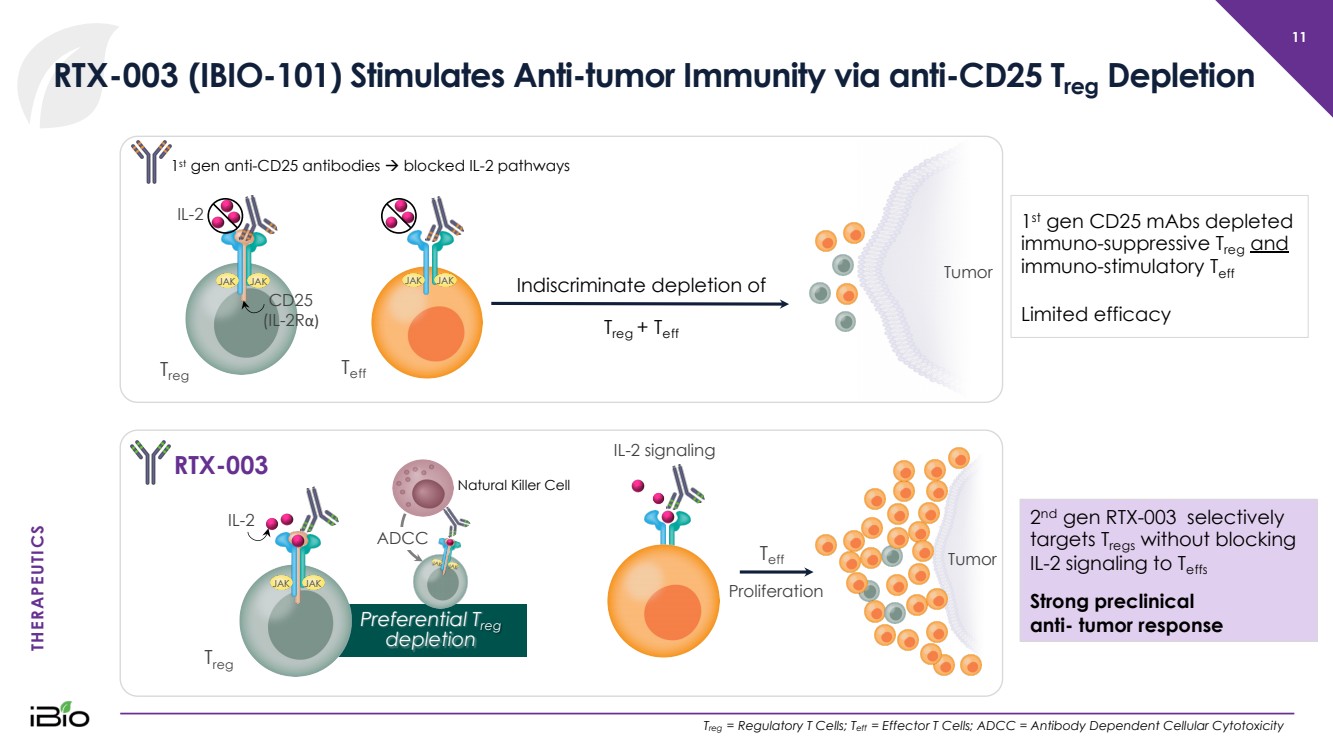
| THERAPEUTICS Natural Killer Cell Indiscriminate depletion of T reg + T eff RTX - 003 (IBIO - 101) Stimulates Anti - tumor Immunity via anti - CD25 T reg Depletion 11 T reg = Regulatory T Cells; T eff = Effector T Cells; ADCC = Antibody Dependent Cellular Cytotoxicity T eff JAK JAK 1 st gen anti - CD25 antibodies à blocked IL - 2 pathways RTX - 003 JAK JAK Tumor Tumor T eff T reg JAK JAK Preferential T reg depletion 1 st gen CD25 mAbs depleted immuno - suppressive T reg and immuno - stimulatory T eff Limited efficacy 2 nd gen RTX - 003 selectively targets T regs without blocking IL - 2 signaling to T effs Strong preclinical anti - tumor response IL - 2 CD25 (IL - 2R α ) JAK JAK IL - 2 T reg IL - 2 signaling Proliferation ADCC JAK JAK |
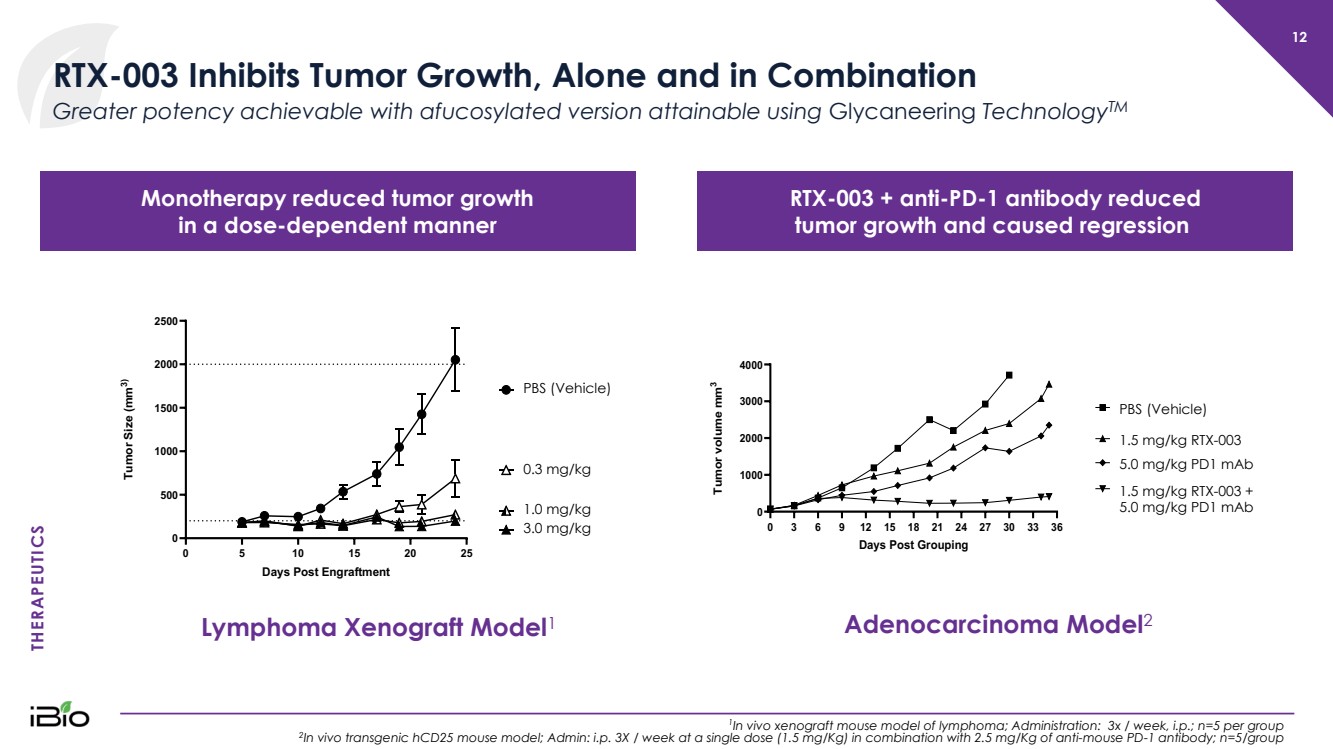
| THERAPEUTICS 12 RTX - 003 Inhibits Tumor Growth, Alone and in Combination 1 In vivo xenograft mouse model of lymphoma; Administration: 3x / week, i.p. ; n=5 per group 2 In vivo transgenic hCD25 mouse model; Admin: i.p. 3X / week at a single dose (1.5 mg/Kg) in combination with 2.5 mg/Kg of anti - mouse PD - 1 antibody; n=5/group Greater potency achievable with afucosylated version attainable using Glycaneering Technology TM 0 5 1 0 1 5 2 0 2 5 0 5 0 0 1 0 0 0 1 5 0 0 2 0 0 0 2 5 0 0 D a y s P o s t E n g r a f t m e n t T u m o r S i z e ( m m 3 ) P B S D 1 1 3 m g / k g D 1 1 1 m g / k g D 1 1 0 .. 3 m g / k g PBS (Vehicle) 0.3 mg/kg 1.0 mg/kg 3.0 mg/kg Lymphoma Xenograft Model 1 Adenocarcinoma Model 2 0 3 6 9 1 2 1 5 1 8 2 1 2 4 2 7 3 0 3 3 3 6 0 1 0 0 0 2 0 0 0 3 0 0 0 4 0 0 0 D a y s P o s t G r o u p i n g T u m o r v o l u m e m m 3 P D 1 5 m g / k g D 1 1 1 .. 5 + P D 1 5 m g / k g P B S D 1 1 1 .. 5 m g / k g PBS (Vehicle) 1.5 mg/kg RTX - 003 5.0 mg/kg PD1 mAb 1.5 mg/kg RTX - 003 + 5.0 mg/kg PD1 mAb Monotherapy reduced tumor growth in a dose - dependent manner RTX - 003 + anti - PD - 1 antibody reduced tumor growth and caused regression |
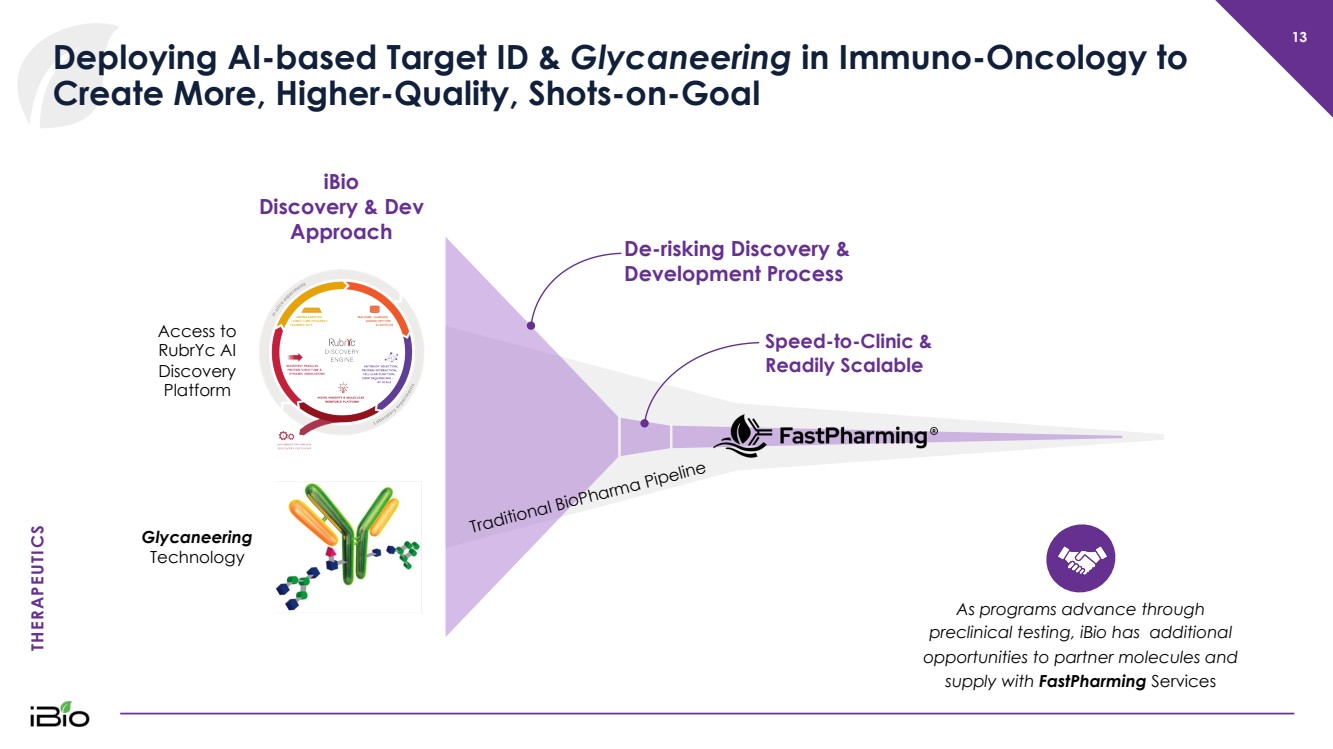
| THERAPEUTICS 13 Deploying AI - based Target ID & Glycaneering in Immuno - Oncology to Create More, Higher - Quality, Shots - on - Goal Speed - to - Clinic & Readily Scalable Access to RubrYc AI Discovery Platform Glycaneering Technology As programs advance through preclinical testing, iBio has additional opportunities to partner molecules and supply with FastPharming Services De - risking Discovery & Development Process iBio Discovery & Dev Approach Traditional BioPharma Pipeline |
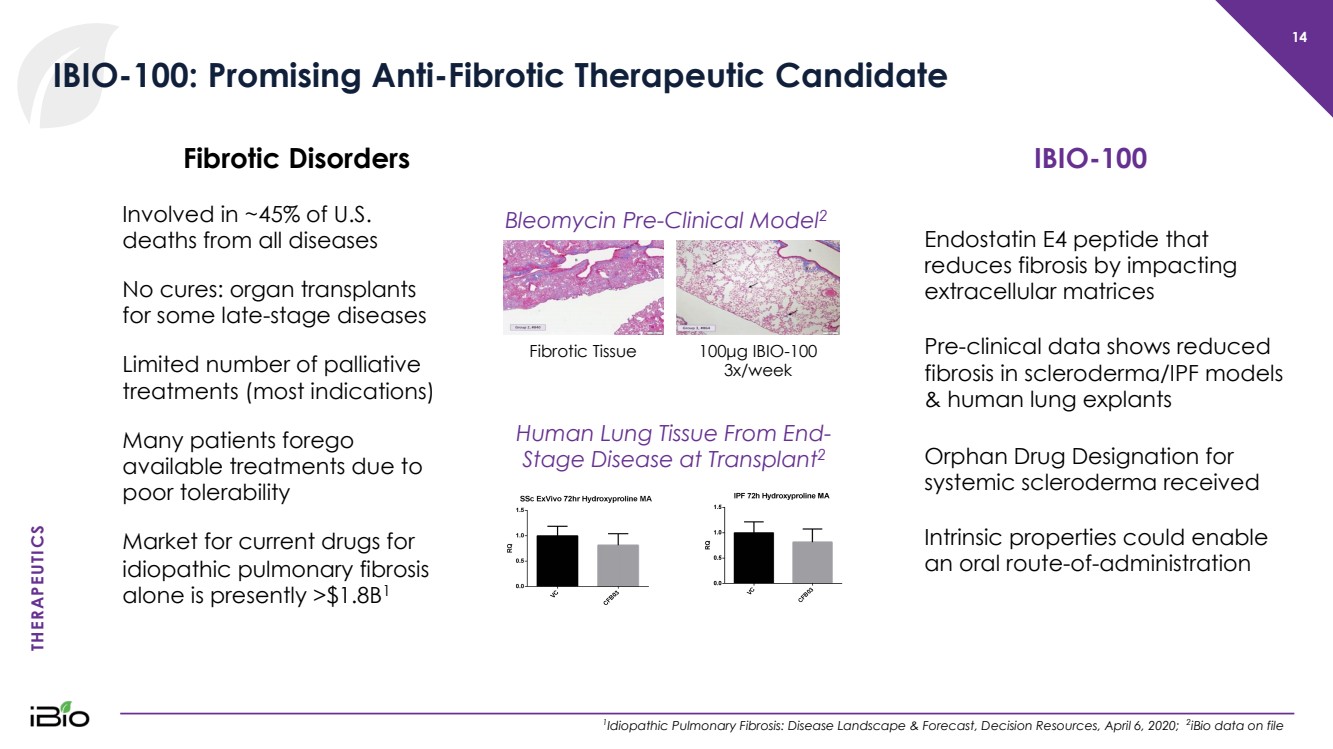
| THERAPEUTICS 14 IBIO - 100: Promising Anti - Fibrotic Therapeutic Candidate 1 Idiopathic Pulmonary Fibrosis: Disease Landscape & Forecast, Decision Resources, April 6, 2020; 2 iBio data on file Involved in ~45% of U.S. deaths from all diseases No cures: organ transplants for some late - stage diseases Limited number of palliative treatments (most indications) Many patients forego available treatments due to poor tolerability Market for current drugs for idiopathic pulmonary fibrosis alone is presently >$1.8B 1 Fibrotic Disorders Endostatin E4 peptide that reduces fibrosis by impacting extracellular matrices Pre - clinical data shows reduced fibrosis in scleroderma/IPF models & human lung explants IBIO - 100 Orphan Drug Designation for systemic scleroderma received Intrinsic properties could enable an oral route - of - administration 100 µ g I BIO - 100 3x/week Fibrotic Tissue Bleomycin Pre - Clinical Model 2 Human Lung Tissue From End - Stage Disease at Transplant 2 |
| Vaccines Human Health: COVID - 19 Animal Health: CSFV |
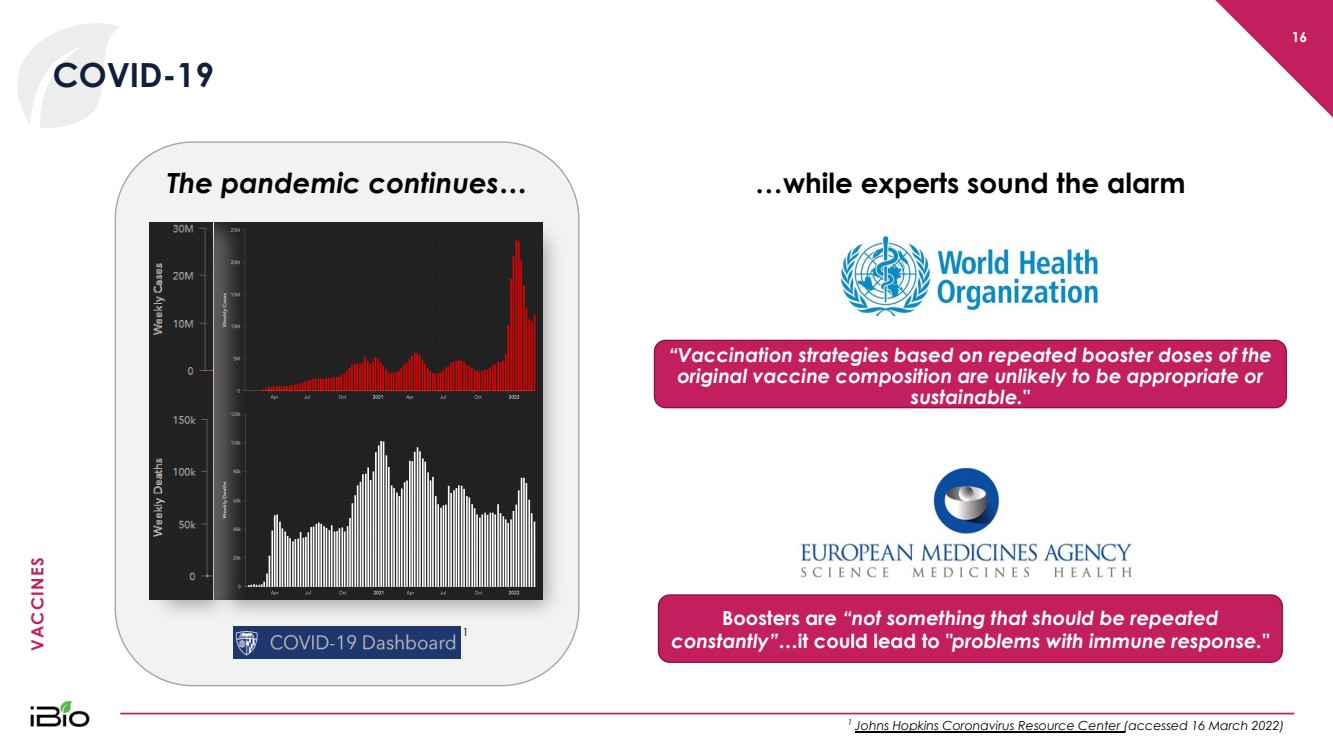
| VACCINES 16 COVID - 19 1 Johns Hopkins Coronavirus Resource Center (accessed 16 March 2022) …while experts sound the alarm “V accination strategies based on repeated booster doses of the original vaccine composition are unlikely to be appropriate or sustainable." Boosters are “not something that should be repeated constantly”… it could lead to "problems with immune response." 1 The pandemic continues… |
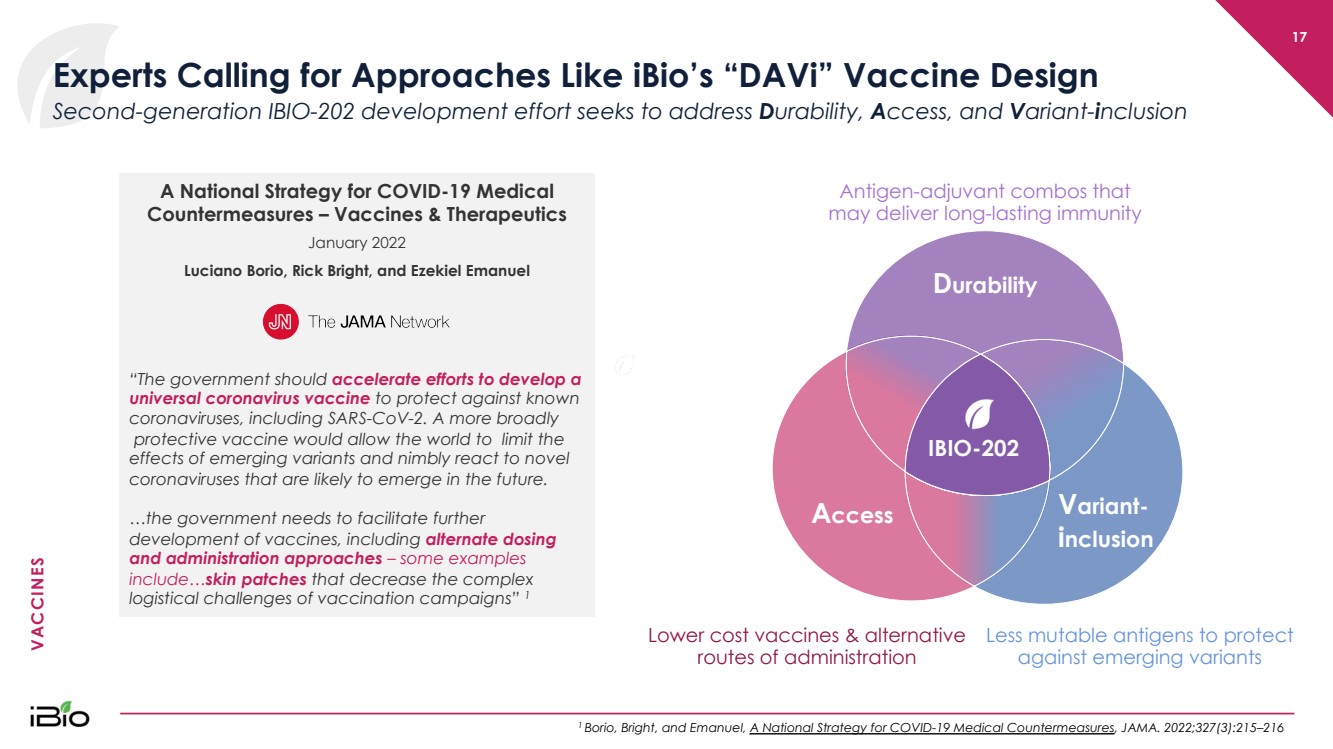
| VACCINES Lower cost vaccines & alternative routes of administration 17 Experts Calling for Approaches Like iBio’s “ DAVi ” Vaccine Design 1 Borio , Bright, and Emanuel, A National Strategy for COVID - 19 Medical Countermeasures , JAMA. 2022;327(3):215 – 216 Second - generation IBIO - 202 development effort seeks to address D urability, A ccess, and V ariant - i nclusion Antigen - adjuvant combos that may deliver long - lasting immunity Less mutable antigens to protect against emerging variants Durability Variant - inclusion Access IBIO - 202 D urability V ariant - i nclusion A ccess A National Strategy for COVID - 19 Medical Countermeasures – Vaccines & Therapeutics January 2022 Luciano Borio , Rick Bright, and Ezekiel Emanuel “The government should accelerate efforts to develop a universal coronavirus vaccine to protect against known coronaviruses, in cluding SARS - CoV - 2. A more broadly protective vaccine would allow the world to limit the effects of emerging variants and nimbly react to novel coronaviruses that are likely to emerge in the future. …the government needs to facilitate further development of vaccines, including alternate dosing and administration approaches – some examples include… skin patches that decrease the complex logistical challenges of vaccination campaigns” 1 |
| VACCINES 18 IBIO - 202 Nucleocapsid [N] - based Subunit Vaccine May Complement to Current and Future Spike [S] - based Vaccines 1 Dai, L. & Gao, G. F. Viral targets for vaccines against COVID - 19. Nature Reviews Immunology 21, 73 – 82 (2021); 2 Fielding CA, et.al .., ADNKA overcomes SARS - CoV2 - mediated NK cell inhibition through non - spike antibodies. bioRxiv , (April 2021) The antigen is produced in our rapidly scalable FastPharming System N - antigen more effective than S in stimulating Natural Killer cell activation 2 Prospectively suitable for delivery via routes other than intramuscular injection N protein function is critical to viral genome packaging and is more highly conserved than the S protein. Thus, new viral variants may be less likely to escape N - based vaccines 1 |
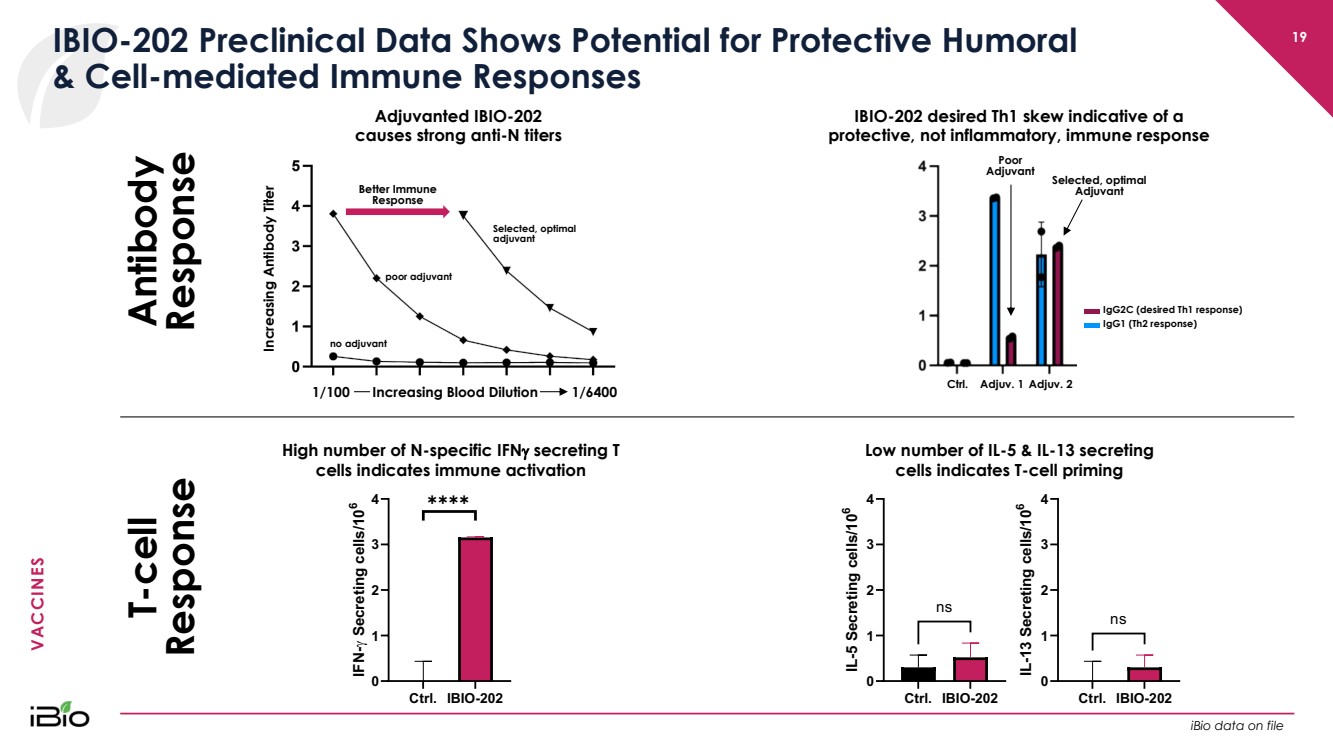
| VACCINES 19 IBIO - 202 Preclinical Data Shows Potential for Protective Humoral & Cell - mediated Immune Responses C t r l .. I B I O - 2 0 2 0 1 2 3 4 I F N - g S e c r e t i n g c e l l s / 1 0 6 ✱✱✱✱ High number of N - specific IFN g secreting T cells indicates immune activation C t r l .. I B I O - 2 0 2 0 1 2 3 4 I L - 5 S e c r e t i n g c e l l s / 1 0 6 n s C t r l .. I B I O - 2 0 2 0 1 2 3 4 I L - 1 3 S e c r e t i n g c e l l s / 1 0 6 n s Low number of IL - 5 & IL - 13 secreting cells indicates T - cell priming Better Immune Response Increasing Antibody Titer 1/100 1/6400 no adjuvant poor adjuvant Selected, optimal adjuvant Increasing Blood Dilution Adjuvanted IBIO - 202 causes strong anti - N titers IBIO - 202 desired Th1 skew indicative of a protective, not inflammatory, immune response Ctrl. Adjuv .. 2 Poor Adjuvant IgG1 (Th2 response) IgG2C (desired Th1 response) Selected, optimal Adjuvant Adjuv .. 1 Antibody Response T - cell Response iBio data on file |
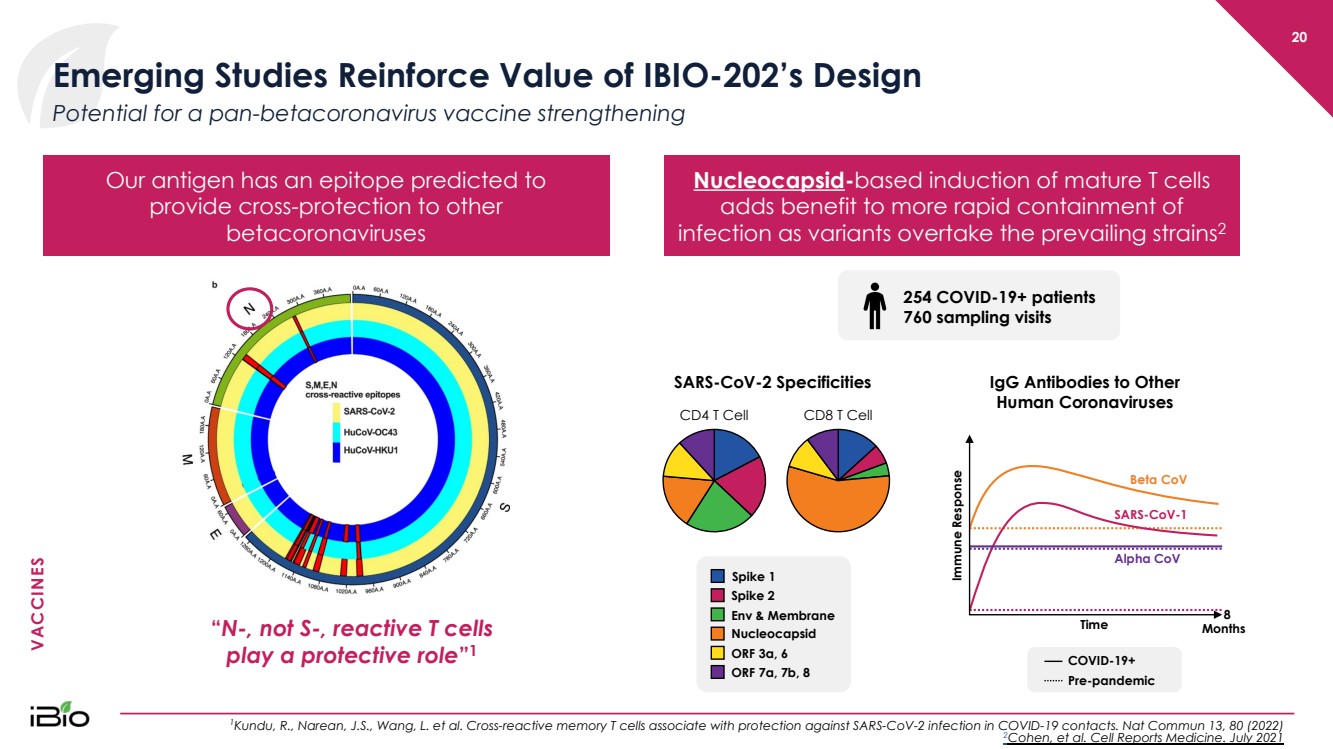
| VACCINES 20 Emerging Studies Reinforce Value of IBIO - 202’s Design 1 Kundu, R., Narean, J.S., Wang, L. et al. Cross - reactive memory T cells associate with protection against SARS - CoV - 2 infection in COVID - 19 contacts. Nat Commun 13, 8 0 (2022) 2 Cohen, et al. Cell Reports Medicine. July 2021 Potential for a pan - betacoronavirus vaccine strengthening Nucleocapsid - based induction of mature T cells adds benefit to more rapid containment of infection as variants overtake the prevailing strains 2 Our antigen has an epitope predicted to provide cross - protection to other betacoronaviruses “ N - , not S - , reactive T cells play a protective role ” 1 IgG Antibodies to Other Human Coronaviruses COVID - 19+ Pre - pandemic Time Immune Response Months 8 Beta CoV SARS - CoV - 1 Alpha CoV 254 COVID - 19+ patients 760 sampling visits SARS - CoV - 2 Specificities CD4 T Cell CD8 T Cell Spike 1 Spike 2 Env & Membrane Nucleocapsid ORF 3a, 6 ORF 7a, 7b, 8 |
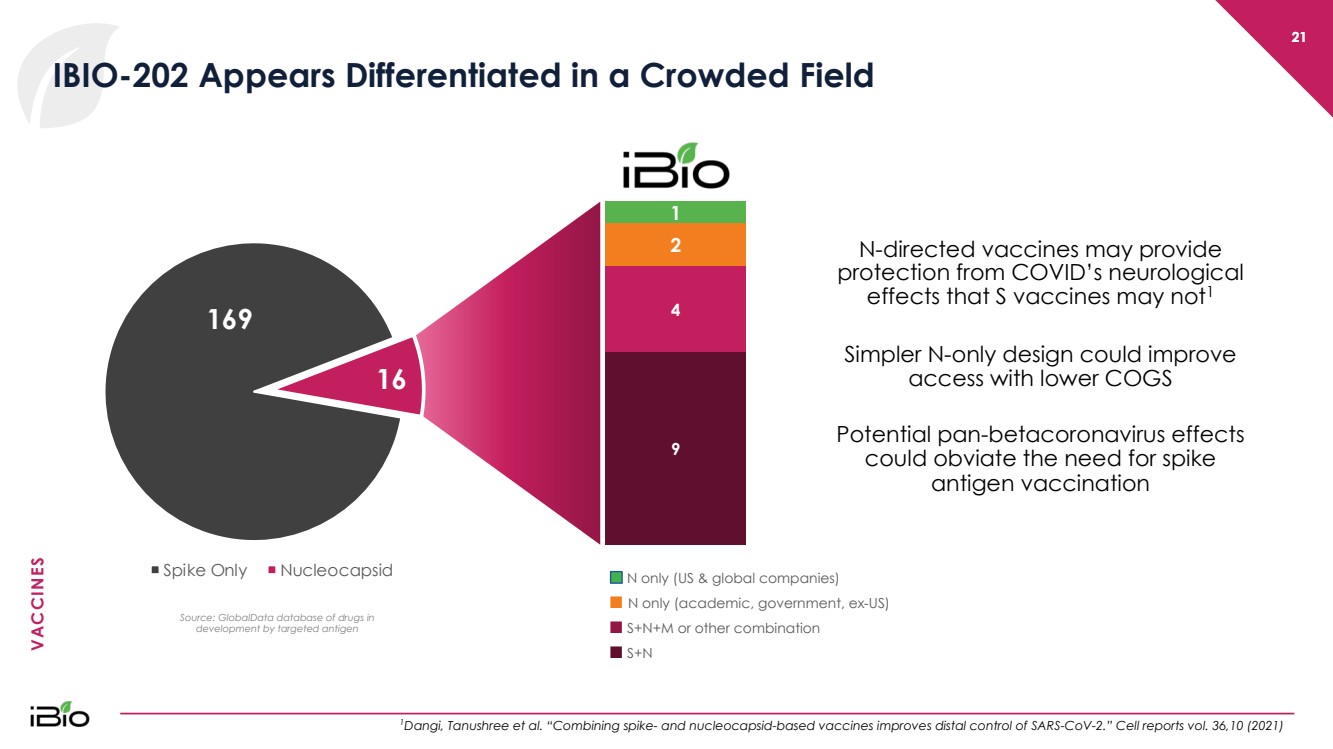
| VACCINES 9 4 2 1 N only (US & global companies) ■ N only (academic, government, ex - US) ■ S+N+M or other combination ■ S+N 21 IBIO - 202 Appears Differentiated in a Crowded Field 1 Dangi, Tanushree et al. “Combining spike - and nucleocapsid - based vaccines improves distal control of SARS - CoV - 2.” Cell reports vol. 36,10 (2021) 21 N - directed vaccines may provide protection from COVID’s neurological effects that S vaccines may not 1 Simpler N - only design could improve access with lower COGS Potential pan - betacoronavirus effects could obviate the need for spike antigen vaccination 169 16 Spike Only Nucleocapsid Source: GlobalData database of drugs in development by targeted antigen |
| Classical Swine Fever Animal Health |
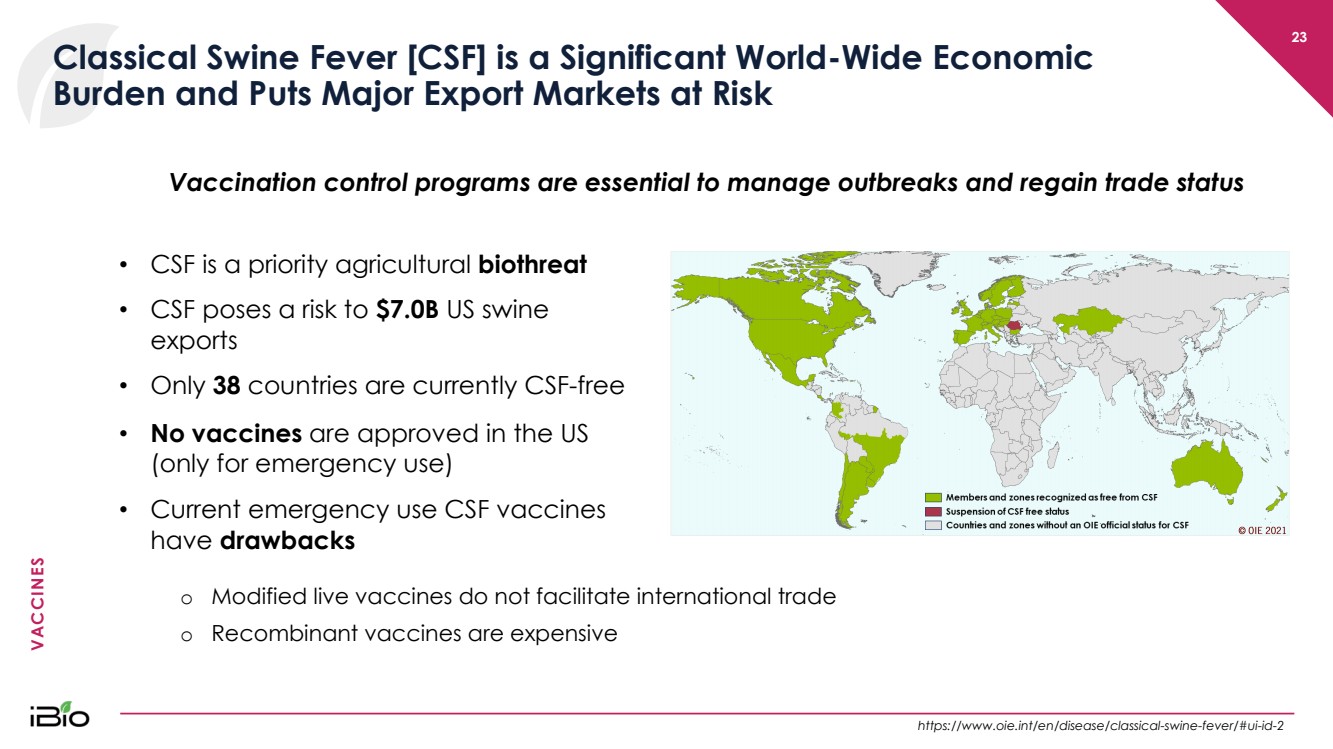
| VACCINES 23 Classical Swine Fever [CSF] is a Significant World - Wide Economic Burden and Puts Major Export Markets at Risk • CSF is a priority agricultural biothreat • CSF poses a risk to $7.0B US swine exports • Only 38 countries are currently CSF - free • No vaccines are approved in the US (only for emergency use) • Current emergency use CSF vaccines have drawbacks Vaccination control programs are essential to manage outbreaks and regain trade status https:// www.oie.int / en /disease/classical - swine - fever/#ui - id - 2 o Modified live vaccines do not facilitate international trade o Recombinant vaccines are expensive |
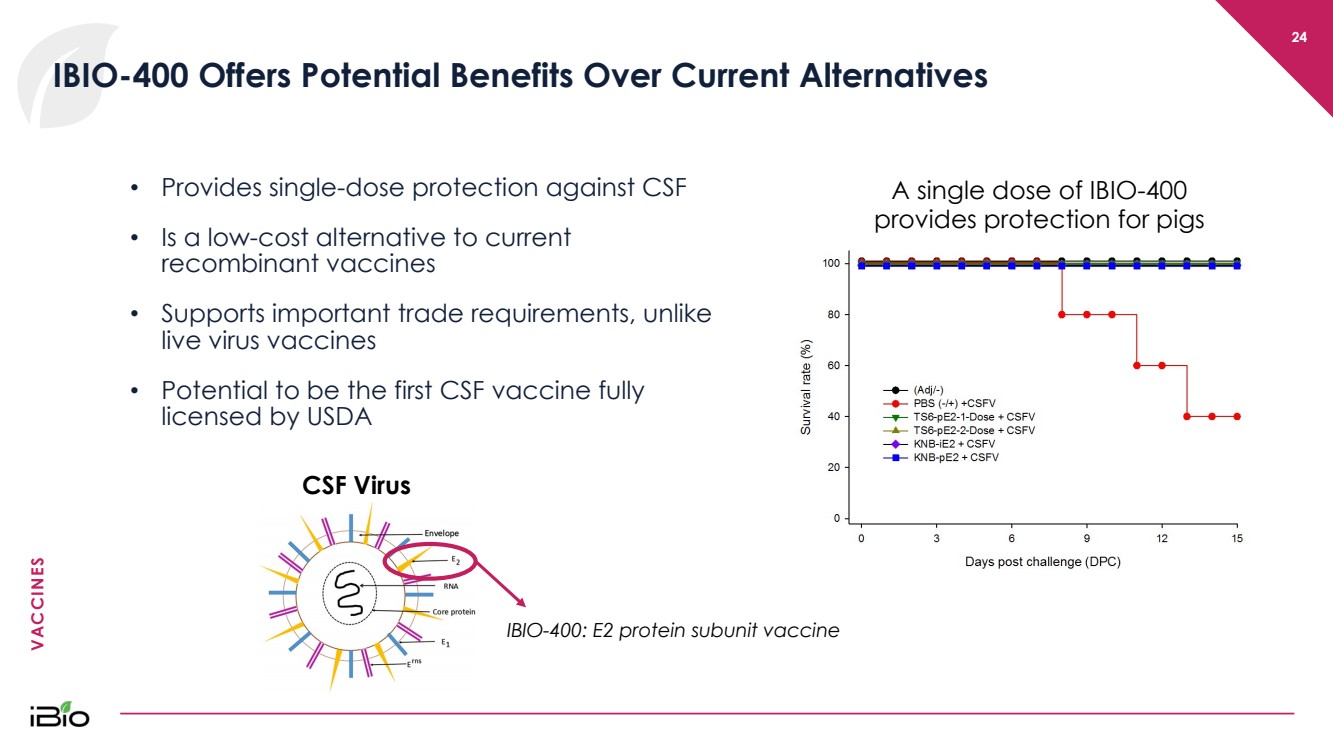
| VACCINES 24 IBIO - 400 Offers Potential Benefits Over Current Alternatives • Provides single - dose protection against CSF • Is a low - cost alternative to current recombinant vaccines • Supports important trade requirements, unlike live virus vaccines • Potential to be the first CSF vaccine fully licensed by USDA CSF Virus A single dose of IBIO - 400 provides protection for pigs IBIO - 400: E2 protein subunit vaccine |
| In Summary |
| Our Leadership Team Brings Drug Development & Bioprocessing Experience 26 Tom Isett CEO & Chairman Martin Brenner , DVM, Ph.D. CSO Robert Lutz, MBA CFBO Randy Maddux , MBA COO Lisa Middlebrook CHRO |
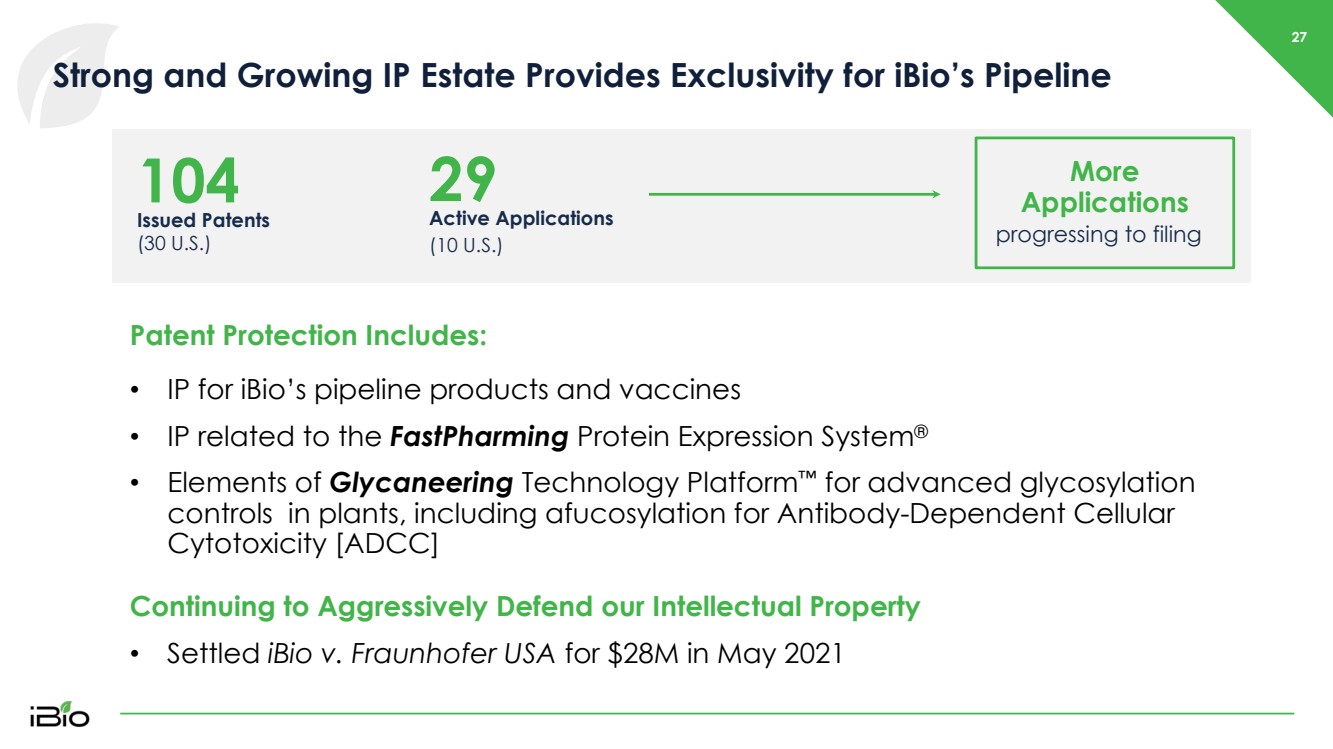
| 27 Strong and Growing IP Estate Provides Exclusivity for iBio’s Pipeline Issued Patents (30 U.S.) 104 Active Applications (10 U.S.) 29 More Applications progressing to filing Patent Protection Includes: • IP for iBio’s pipeline products and vaccines • IP related to the FastPharming Protein Expression System ® • Elements of Glycaneering Technology Platform ™ for advanced glycosylation controls in plants, including afucosylation for Antibody - Dependent Cellular Cytotoxicity [ADCC] Continuing to Aggressively Defend our Intellectual Property • Settled iBio v. Fraunhofer USA for $28M in May 2021 |
| 28 Financial Overview § Publicly traded (NYSEA: IBIO) since Jan 2008 § Approximately $57.4M in cash and cash equivalents plus investments in debt securities, excluding $5.9M of restricted cash (31 Dec 2021) § Approximately 218.0M common shares & 17M options, restricted stock units and warrants outstanding (31 Dec 2021) § Texas Manufacturing Facility § Purchased in Nov 2021 with approximately $22.4M debt (secured by the facility), $6M in cash, and 1.3M warrants § Evaluating sale - leaseback to extinguish the debt and recover cash § Current cash provides runway through Sept 30, 2023 |
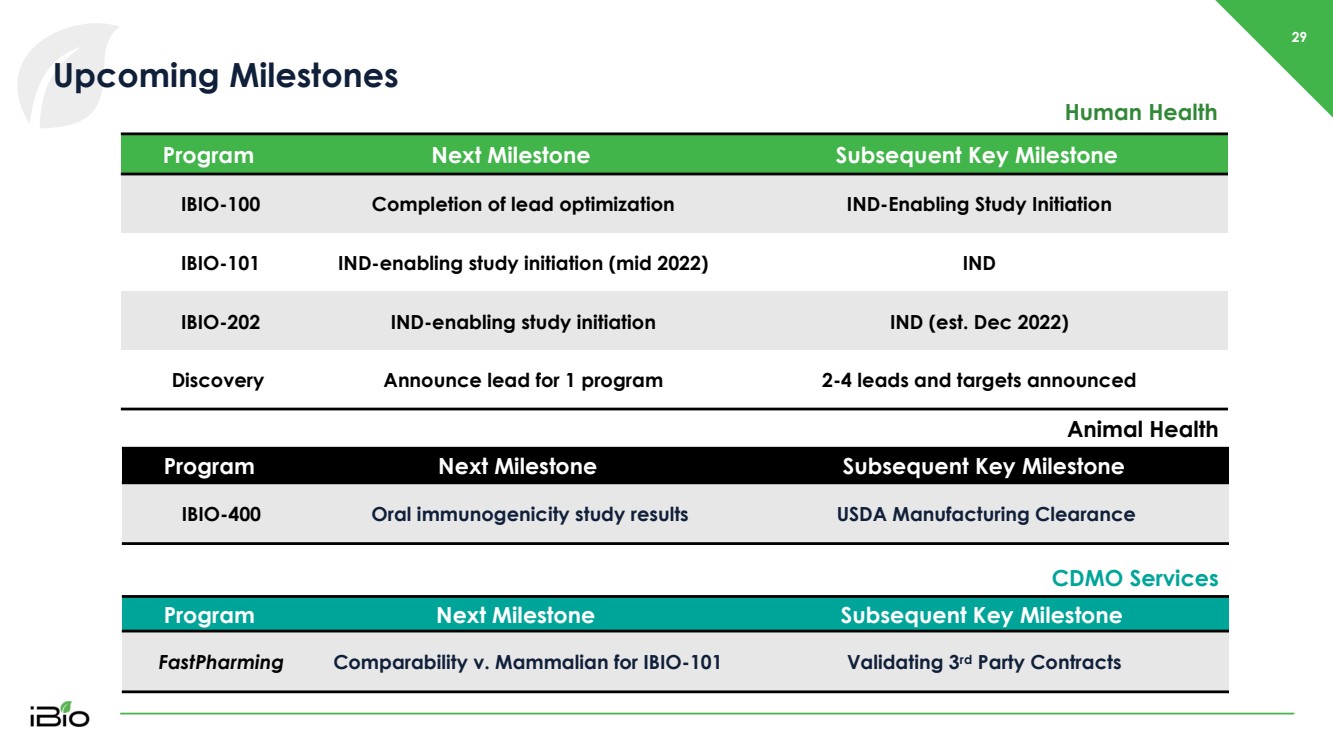
| 29 Upcoming Milestones Human Health Program Next Milestone Subsequent Key Milestone IBIO - 400 Oral immunogenicity study results USDA Manufacturing Clearance Program Next Milestone Subsequent Key Milestone IBIO - 100 Completion of lead optimization IND - Enabling Study Initiation IBIO - 101 IND - enabling study initiation (mid 2022) IND IBIO - 202 IND - enabling study initiation IND (est. Dec 2022) Discovery Announce lead for 1 program 2 - 4 leads and targets announced Animal Health Program Next Milestone Subsequent Key Milestone FastPharming Comparability v. Mammalian for IBIO - 101 Validating 3 rd Party Contracts CDMO Services |
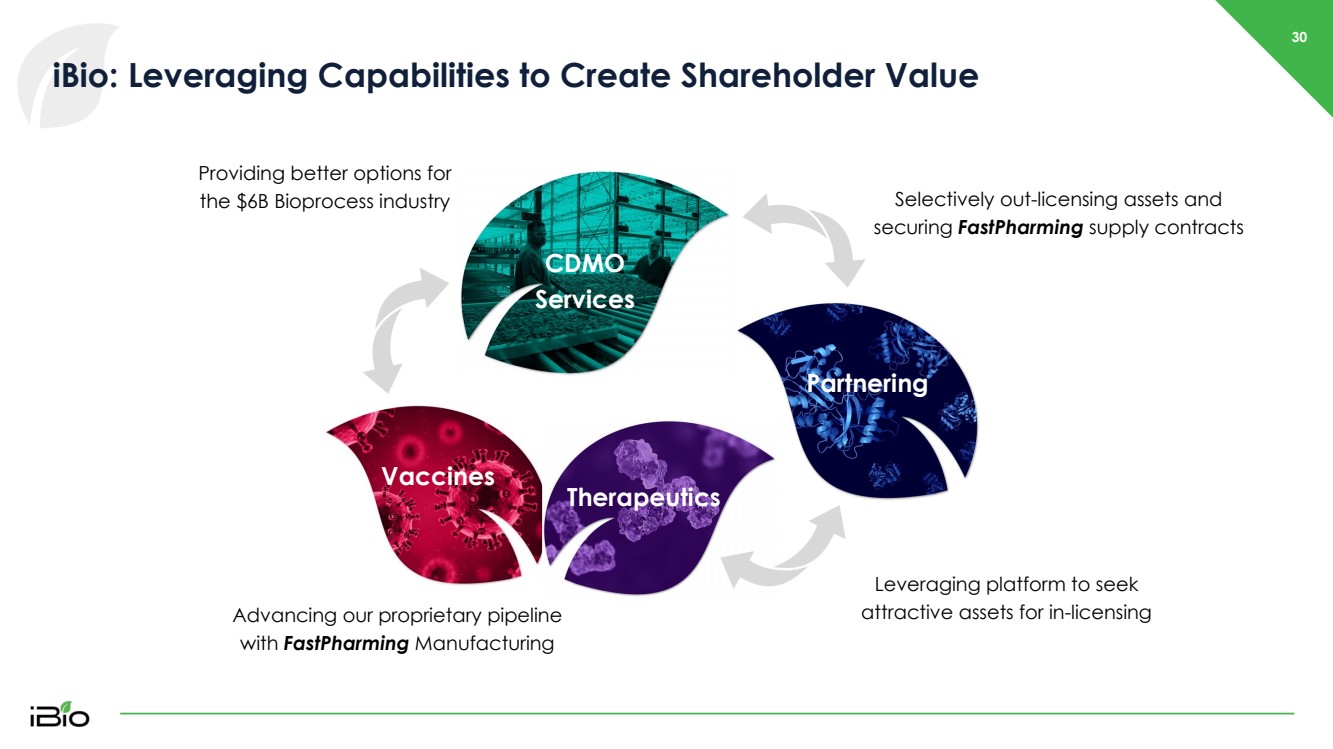
| 30 iBio : Leveraging Capabilities to Create Shareholder Value Advancing our proprietary pipeline with FastPharming Manufacturing Partnering Providing better options for the $6B Bioprocess industry CDMO Services Vaccines Therapeutics Partnering Leveraging platform to seek attractive assets for in - licensing Therapeutics Selectively out - licensing assets and securing FastPharming supply contracts |